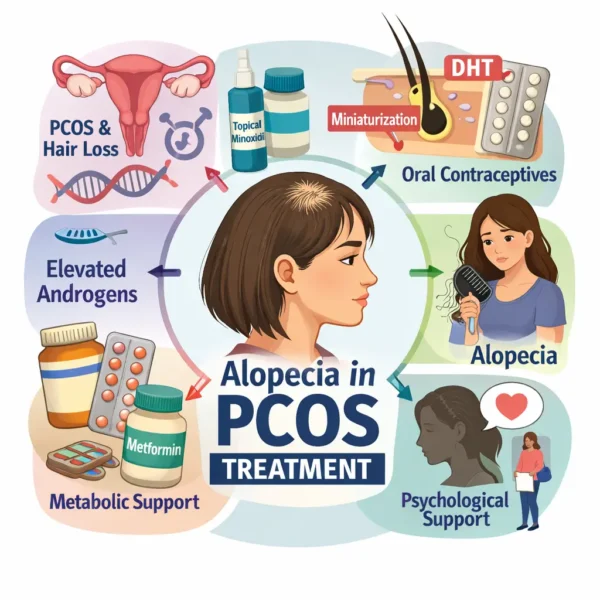
Polycystic ovary syndrome (PCOS) is a highly prevalent endocrine disorder of reproductive-aged women, characterized by hyperandrogenism, ovulatory dysfunction, and polycystic ovarian morphology. In addition to metabolic and reproductive sequelae, PCOS frequently presents with dermatologic manifestations, including acne, hirsutism, and alopecia. Hair loss in PCOS most commonly presents as female pattern hair loss (FPHL), a diffuse, non-scarring alopecia driven by follicular miniaturization. Although FPHL is common in the general female population, its presentation in PCOS patients is clinically distinct, often occurring at a younger age and in association with other signs of androgen excess. More details on the prevalence and biology of hair loss associated with PCOS can be found on this page .
Management of alopecia in PCOS requires a nuanced, multidisciplinary approach that accounts for hormonal pathophysiology, metabolic risk, reproductive considerations, and the psychosocial burden of hair loss. Standard therapies for FPHL may be effective, but treatment selection and sequencing must be adapted to the unique endocrine milieu of PCOS patients. This article reviews current evidence-based treatment strategies for alopecia in PCOS, emphasizing pharmacologic interventions, practical considerations, and emerging therapeutic directions, drawing on recent expert consensus and clinical literature.
Pathophysiologic Rationale for Treatment: Hair loss in PCOS is primarily androgen mediated. Elevated circulating androgens, reduced sex hormone–binding globulin (SHBG), and increased local conversion of testosterone to dihydrotestosterone (DHT) within the scalp all contribute to follicular miniaturization. Importantly, PCOS illustrates the paradox of androgen action: while androgens promote terminal hair growth in androgen-sensitive body sites (hirsutism), they induce progressive miniaturization of scalp hair follicles.
This duality makes clear why effective treatment strategies must reduce systemic androgen levels, block androgen receptor activity, inhibit local androgen metabolism, or stimulate follicular growth through androgen-independent pathways. In practice, many PCOS patients with alopecia require combination therapy targeting multiple mechanisms simultaneously.
General Principles of Treatment: Several overarching principles guide the treatment of alopecia in PCOS:
Early intervention is important, as follicular miniaturization becomes increasingly irreversible over time.Combination therapy is often required, as monotherapy rarely addresses both androgen excess and follicular responsiveness.Reproductive safety must be carefully considered, as many effective antiandrogen therapies are teratogenic.Metabolic comorbidities and concurrent symptoms such as acne and hirsutism should influence therapeutic choice.Long-term adherence is essential; most therapies require months to demonstrate benefit and must be continued indefinitely to maintain results. Topical Therapies – Topical Minoxidil: Topical minoxidil remains the first-line therapy for FPHL, including in PCOS patients. It is the only FDA-approved treatment for female pattern hair loss and has a well-established safety and efficacy profile. Minoxidil promotes hair growth by prolonging the anagen phase and increasing follicular size through mechanisms independent of androgen signaling.
In PCOS patients, however, topical minoxidil presents a particular challenge due to the risk of facial hypertrichosis, which may exacerbate pre-existing hirsutism. Although this side effect occurs in a minority of patients, it can be distressing and contribute to treatment discontinuation. Strategies to mitigate this risk include careful application technique, avoidance of facial runoff, and concurrent use of systemic antiandrogens.
Despite these concerns, topical minoxidil remains foundational therapy and is frequently combined with hormonal or antiandrogen treatments to enhance efficacy.
Systemic Therapies – Combined Oral Contraceptives: Combined oral contraceptive pills (COCs) are central to the management of PCOS and play an important role in treating alopecia. Their benefit derives primarily from estrogen-mediated increases in SHBG, leading to reduced free androgen levels, as well as suppression of ovarian androgen production.
Not all COCs are equivalent in this context. Preparations containing progestins with high androgenic activity may worsen hair loss and are generally avoided. Third- and fourth-generation COCs, particularly those containing drospirenone or cyproterone acetate, are preferred due to their antiandrogenic or minimally androgenic profiles.
COCs may improve hair density and slow progression of alopecia, particularly when iron stores are adequate. However, clinicians must counsel patients regarding the potential for transient telogen effluvium following initiation or discontinuation, as well as mood-related side effects. In appropriately selected patients, COCs provide both therapeutic and contraceptive benefit, making them especially valuable when combined with other teratogenic agents.
Antiandrogens – Spironolactone: Spironolactone is the most widely used systemic antiandrogen for FPHL in women and is particularly well suited for PCOS patients. It acts through androgen receptor antagonism and partial inhibition of 5-alpha reductase.
Spironolactone is effective for alopecia, acne, and hirsutism, making it an attractive option for PCOS patients with multiple androgen-mediated symptoms. Adverse effects include menstrual irregularities, hypotension, and, rarely, hyperkalemia. In healthy young women without renal disease, routine potassium monitoring is generally not required, though practice patterns vary.
Spironolactone is contraindicated in pregnancy due to the risk of feminization of a male fetus, and so reliable contraception is mandatory during treatment with spironolactone.
Nonsteroidal Androgen Receptor Antagonists: Bicalutamide and flutamide are potent androgen receptor antagonists originally developed for prostate cancer. Both have demonstrated efficacy in treating hyperandrogenic alopecia, but their use is generally reserved for refractory cases due to safety concerns.
Bicalutamide, at low doses, has gained interest because it does not inhibit 5-alpha reductase and lacks mineralocorticoid activity. However, hepatotoxicity remains a concern, and periodic liver function monitoring is required when using this drug. Flutamide is less commonly used due to its higher risk of severe liver injury.
These agents are only considered in carefully selected patients who have failed first-line therapies and who can adhere to strict monitoring and contraception requirements.
5-Alpha Reductase Inhibitors: Finasteride and dutasteride inhibit the conversion of testosterone to DHT and are effective treatments for androgen-mediated hair loss. Although they are not officially FDA-approved for use in women, they are commonly prescribed off-label by dermatologists for treating FPHL, including in PCOS patients.
Women with PCOS often require higher doses than men to achieve clinical benefit. Dutasteride, which inhibits both type I and type II 5-alpha reductase, may be more potent but has a much longer half-life, extending the duration of teratogenic risk after discontinuation.
Again, these agents should not be used in women who are pregnant, attempting conception, or unwilling to use reliable contraception. They should also be avoided in patients with a history of hormone-sensitive malignancy.
Oral Minoxidil: Low-dose oral minoxidil has emerged as an alternative to topical therapy, offering improved adherence and consistent systemic delivery. Some smaller studies suggests it is effective for FPHL but it carries a higher risk of generalized hypertrichosis, which is particularly relevant in PCOS patients.
Careful dose titration and patient counseling are essential. Oral minoxidil is sometimes used in combination with antiandrogens to counteract unwanted hair growth in non-scalp areas.
Metabolic Modulation – Metformin: Metformin is widely used in PCOS to improve insulin sensitivity and reduce ovarian androgen production. Although data directly linking metformin to improvement in alopecia are limited, its indirect antiandrogenic effects and favorable metabolic profile make it a valuable adjunctive therapy.
Emerging experimental evidence suggests that metformin may also exert some direct effects on hair follicle biology, including activation of growth-promoting signaling pathways. While metformin alone is unlikely to reverse alopecia, it may enhance overall treatment response when combined with hormonal or antiandrogen therapies.
Psychosocial Considerations: Hair loss is a significant source of psychological distress, and this burden is often amplified in PCOS patients, who already experience higher rates of anxiety, depression, and reduced quality of life. Effective management of alopecia must therefore include acknowledgment of emotional impact, realistic expectation setting, and, when appropriate, referral for psychological support.
Conclusion: Treatment of alopecia in patients with PCOS requires an integrated, individualized approach that addresses both androgen excess and follicular dysfunction while accounting for reproductive and metabolic considerations. Topical minoxidil remains foundational therapy, but most patients benefit from combination treatment incorporating hormonal contraceptives, systemic antiandrogens, or metabolic modulators. As evidence continues to evolve, earlier intervention and personalized regimens offer the greatest potential to preserve hair density and improve quality of life in this challenging patient population.
Bibliography
11711645 {11711645:6QJLUINJ},{11711645:57WEALKF},{11711645:F2DCWVP4},{11711645:655XIQZK},{11711645:UMQ4CZP2},{11711645:L8VJVDZ7},{11711645:QL9K7MKK},{11711645:XZ777L7R},{11711645:X8DIRN4F},{11711645:QMJKTYY2},{11711645:PURXBVQY} 1 vancouver 50 date asc 2036 https://www.keratin.com/wp-content/plugins/zotpress/ %7B%22status%22%3A%22success%22%2C%22updateneeded%22%3Afalse%2C%22instance%22%3Afalse%2C%22meta%22%3A%7B%22request_last%22%3A0%2C%22request_next%22%3A0%2C%22used_cache%22%3Atrue%7D%2C%22data%22%3A%5B%7B%22key%22%3A%22655XIQZK%22%2C%22library%22%3A%7B%22id%22%3A11711645%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Sinclair%20et%20al.%22%2C%22parsedDate%22%3A%222005-03%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%201.35%3B%20%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%20style%3D%26quot%3Bclear%3A%20left%3B%20%26quot%3B%26gt%3B%5Cn%20%20%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-left-margin%26quot%3B%20style%3D%26quot%3Bfloat%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%26quot%3B%26gt%3B1.%26lt%3B%5C%2Fdiv%26gt%3B%26lt%3Bdiv%20class%3D%26quot%3Bcsl-right-inline%26quot%3B%20style%3D%26quot%3Bmargin%3A%200%20.4em%200%201.5em%3B%26quot%3B%26gt%3BSinclair%20R%2C%20Wewerinke%20M%2C%20Jolley%20D.%20Treatment%20of%20female%20pattern%20hair%20loss%20with%20oral%20antiandrogens.%20Br%20J%20Dermatol.%202005%20Mar%3B152%283%29%3A466%26%23x2013%3B73.%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%20%20%20%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Treatment%20of%20female%20pattern%20hair%20loss%20with%20oral%20antiandrogens%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22R.%22%2C%22lastName%22%3A%22Sinclair%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22M.%22%2C%22lastName%22%3A%22Wewerinke%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22D.%22%2C%22lastName%22%3A%22Jolley%22%7D%5D%2C%22abstractNote%22%3A%22BACKGROUND%3A%20It%20has%20not%20been%20conclusively%20established%20that%20female%20pattern%20hair%20loss%20%28FPHL%29%20is%20either%20due%20to%20androgens%20or%20responsive%20to%20oral%20antiandrogen%20therapy.%5CnOBJECTIVES%3A%20To%20evaluate%20the%20efficacy%20of%20oral%20antiandrogen%20therapy%20in%20the%20management%20of%20women%20with%20FPHL%20using%20standardized%20photographic%20techniques%20%28Canfield%20Scientific%29%2C%20and%20to%20identify%20clinical%20and%20histological%20parameters%20predictive%20of%20clinical%20response.%5CnMETHODS%3A%20For%20this%20single-centre%2C%20before-after%2C%20open%20intervention%20study%2C%2080%20women%20aged%20between%2012%20and%2079%20years%2C%20with%20FPHL%20and%20biopsy-confirmed%20hair%20follicle%20miniaturization%20%5Bterminal%5C%2Fvellus%20%28T%5C%2FV%29%20hair%20ratio%20%26lt%3B%20or%20%3D%204%20%3A%201%5D%20were%20photographed%20at%20baseline%20and%20again%20after%20receiving%20a%20minimum%20of%2012%20months%20of%20oral%20antiandrogen%20therapy.%20Forty%20women%20received%20spironolactone%20200%20mg%20daily%20and%2040%20women%20received%20cyproterone%20acetate%2C%20either%2050%20mg%20daily%20or%20100%20mg%20for%2010%20days%20per%20month%20if%20premenopausal.%20Women%20using%20topical%20minoxidil%20were%20excluded.%20Standardized%20photographs%20of%20the%20midfrontal%20and%20vertex%20scalp%20were%20taken%20with%20the%20head%20positioned%20in%20a%20stereotactic%20device.%20Images%20were%20evaluated%20by%20a%20panel%20of%20three%20clinicians%20experienced%20in%20the%20assessment%20of%20FPHL%2C%20blinded%20to%20patient%20details%20and%20treatment%20and%20using%20a%20three-point%20scale.%5CnRESULTS%3A%20As%20there%20was%20no%20significant%20difference%20in%20the%20results%20or%20the%20trend%20between%20spironolactone%20and%20cyproterone%20acetate%20the%20results%20were%20combined.%20Thirty-five%20%2844%25%29%20women%20had%20hair%20regrowth%2C%2035%20%2844%25%29%20had%20no%20clear%20change%20in%20hair%20density%20before%20and%20after%20treatment%2C%20and%2010%20%2812%25%29%20experienced%20continuing%20hair%20loss%20during%20the%20treatment%20period.%20Ordinal%20logistic%20regression%20analysis%20to%20identify%20predictors%20of%20response%20revealed%20no%20influence%20of%20patient%20age%2C%20menopause%20status%2C%20serum%20ferritin%2C%20serum%20hormone%20levels%2C%20clinical%20stage%20%28Ludwig%29%20or%20histological%20parameters%20such%20as%20T%5C%2FV%20ratio%20or%20fibrosis.%20The%20only%20significant%20predictor%20was%20midscalp%20clinical%20grade%2C%20with%20higher-scale%20values%20associated%20with%20a%20greater%20response%20%28P%20%3D%200.013%29.%5CnCONCLUSION%3A%20Eighty-eight%20percent%20of%20women%20receiving%20oral%20antiandrogens%20could%20expect%20to%20see%20no%20progression%20of%20their%20FPHL%20or%20improvement.%20High%20midscalp%20clinical%20grade%20was%20the%20only%20predictor%20of%20response%20identified.%20A%20placebo-controlled%20study%20is%20required%20to%20compare%20this%20outcome%20to%20the%20natural%20history%20of%20FPHL.%22%2C%22date%22%3A%222005-03%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%2210.1111%5C%2Fj.1365-2133.2005.06218.x%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%220007-0963%22%2C%22language%22%3A%22eng%22%2C%22collections%22%3A%5B%2286XZQUNS%22%5D%2C%22dateModified%22%3A%222026-01-18T15%3A31%3A35Z%22%7D%7D%2C%7B%22key%22%3A%22F2DCWVP4%22%2C%22library%22%3A%7B%22id%22%3A11711645%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Hoedemaker%20et%20al.%22%2C%22parsedDate%22%3A%222007-02%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%201.35%3B%20%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%20style%3D%26quot%3Bclear%3A%20left%3B%20%26quot%3B%26gt%3B%5Cn%20%20%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-left-margin%26quot%3B%20style%3D%26quot%3Bfloat%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%26quot%3B%26gt%3B1.%26lt%3B%5C%2Fdiv%26gt%3B%26lt%3Bdiv%20class%3D%26quot%3Bcsl-right-inline%26quot%3B%20style%3D%26quot%3Bmargin%3A%200%20.4em%200%201.5em%3B%26quot%3B%26gt%3BHoedemaker%20C%2C%20van%20Egmond%20S%2C%20Sinclair%20R.%20Treatment%20of%20female%20pattern%20hair%20loss%20with%20a%20combination%20of%20spironolactone%20and%20minoxidil.%20Australas%20J%20Dermatol.%202007%20Feb%3B48%281%29%3A43%26%23x2013%3B5.%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%20%20%20%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Treatment%20of%20female%20pattern%20hair%20loss%20with%20a%20combination%20of%20spironolactone%20and%20minoxidil%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Carlijn%22%2C%22lastName%22%3A%22Hoedemaker%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sylvia%22%2C%22lastName%22%3A%22van%20Egmond%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Rodney%22%2C%22lastName%22%3A%22Sinclair%22%7D%5D%2C%22abstractNote%22%3A%22A%2053-year-old%20woman%20with%20clinical%20evidence%20of%20female%20pattern%20hair%20loss%20and%20histological%20evidence%20of%20androgenetic%20alopecia%20was%20initially%20treated%20with%20the%20oral%20antiandrogen%20spironolactone%20200%20mg%20daily.%20Serial%20scalp%20photography%20documented%20hair%20regrowth%20at%2012%20months%3B%20however%2C%20the%20hair%20regrowth%20plateaued%2C%20and%20at%2024%20months%20there%20had%20been%20no%20further%20improvement%20in%20hair%20density.%20Twice%20daily%20therapy%20with%20topical%20minoxidil%205%25%20solution%20was%20then%20introduced%20and%20further%20regrowth%20documented%2C%20confirming%20the%20additive%20effect%20of%20combination%20therapy.%22%2C%22date%22%3A%222007-02%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%2210.1111%5C%2Fj.1440-0960.2007.00332.x%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%220004-8380%22%2C%22language%22%3A%22eng%22%2C%22collections%22%3A%5B%2286XZQUNS%22%5D%2C%22dateModified%22%3A%222026-01-18T15%3A31%3A52Z%22%7D%7D%2C%7B%22key%22%3A%22UMQ4CZP2%22%2C%22library%22%3A%7B%22id%22%3A11711645%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Rathnayake%20and%20Sinclair%22%2C%22parsedDate%22%3A%222010-07%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%201.35%3B%20%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%20style%3D%26quot%3Bclear%3A%20left%3B%20%26quot%3B%26gt%3B%5Cn%20%20%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-left-margin%26quot%3B%20style%3D%26quot%3Bfloat%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%26quot%3B%26gt%3B1.%26lt%3B%5C%2Fdiv%26gt%3B%26lt%3Bdiv%20class%3D%26quot%3Bcsl-right-inline%26quot%3B%20style%3D%26quot%3Bmargin%3A%200%20.4em%200%201.5em%3B%26quot%3B%26gt%3BRathnayake%20D%2C%20Sinclair%20R.%20Innovative%20use%20of%20spironolactone%20as%20an%20antiandrogen%20in%20the%20treatment%20of%20female%20pattern%20hair%20loss.%20Dermatol%20Clin.%202010%20July%3B28%283%29%3A611%26%23x2013%3B8.%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%20%20%20%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Innovative%20use%20of%20spironolactone%20as%20an%20antiandrogen%20in%20the%20treatment%20of%20female%20pattern%20hair%20loss%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Deepani%22%2C%22lastName%22%3A%22Rathnayake%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Rodney%22%2C%22lastName%22%3A%22Sinclair%22%7D%5D%2C%22abstractNote%22%3A%22Patterned%20hair%20loss%20in%20men%20and%20women%2C%20although%20medically%20benign%2C%20is%20a%20common%2C%20albeit%20unwelcome%2C%20event%20that%20may%20cause%20considerable%20anxiety%20and%20concern.%20Patterned%20hair%20loss%20is%20progressive%20and%20when%20untreated%20leads%20to%20baldness.%20The%20prevalence%20and%20severity%20of%20this%20physiologic%20process%20both%20increase%20with%20advancing%20age.%20Although%20androgens%20play%20a%20key%20role%20in%20the%20pathogenesis%20of%20male%20pattern%20hair%20loss%20%28MPHL%29%2C%20the%20role%20of%20androgens%20in%20female%20pattern%20hair%20loss%20%28FPHL%29%20is%20less%20well%20established.%20Satisfactory%20treatment%20response%20to%20antiandrogen%20therapy%20supports%20the%20involvement%20of%20androgens%20in%20the%20pathogenesis%20of%20FPHL.%20Spironolactone%20has%20been%20used%20for%2030%20years%20as%20a%20potassium-sparing%20diuretic.%20Spironolactone%20is%20a%20synthetic%20steroid%20structurally%20related%20to%20aldosterone.%20Since%20the%20serendipitous%20discovery%2020%20years%20ago%20that%20spironolactone%20given%20to%20a%20woman%20for%20polycystic%20ovary%20syndrome%20%28PCOS%29%20and%20associated%20hypertension%20also%20improved%20hirsutism%2C%20it%20has%20been%20used%20as%20a%20primary%20medical%20treatment%20for%20hirsutism.%20Spironolactone%20both%20reduces%20adrenal%20androgen%20production%20and%20exerts%20competitive%20blockade%20on%20androgen%20receptors%20in%20target%20tissues.%20Spironolactone%20has%20been%20used%20off-label%20in%20FPHL%20for%20over%2020%20years.%20It%20has%20been%20shown%20to%20arrest%20hair%20loss%20progression%20with%20a%20long-term%20safety%20profile.%20A%20significant%20percentage%20of%20women%20also%20achieve%20partial%20hair%20regrowth.%20Spironolactone%20is%20not%20used%20in%20male%20androgenetic%20alopecia%20because%20of%20the%20risk%20of%20feminization.%22%2C%22date%22%3A%222010-07%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.det.2010.03.011%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%221558-0520%22%2C%22language%22%3A%22eng%22%2C%22collections%22%3A%5B%2286XZQUNS%22%5D%2C%22dateModified%22%3A%222026-01-18T15%3A30%3A09Z%22%7D%7D%2C%7B%22key%22%3A%22L8VJVDZ7%22%2C%22library%22%3A%7B%22id%22%3A11711645%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Schmidt%20and%20Shinkai%22%2C%22parsedDate%22%3A%222015-10%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%201.35%3B%20%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%20style%3D%26quot%3Bclear%3A%20left%3B%20%26quot%3B%26gt%3B%5Cn%20%20%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-left-margin%26quot%3B%20style%3D%26quot%3Bfloat%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%26quot%3B%26gt%3B1.%26lt%3B%5C%2Fdiv%26gt%3B%26lt%3Bdiv%20class%3D%26quot%3Bcsl-right-inline%26quot%3B%20style%3D%26quot%3Bmargin%3A%200%20.4em%200%201.5em%3B%26quot%3B%26gt%3BSchmidt%20TH%2C%20Shinkai%20K.%20Evidence-based%20approach%20to%20cutaneous%20hyperandrogenism%20in%20women.%20J%20Am%20Acad%20Dermatol.%202015%20Oct%3B73%284%29%3A672%26%23x2013%3B90.%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%20%20%20%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Evidence-based%20approach%20to%20cutaneous%20hyperandrogenism%20in%20women%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Timothy%20H.%22%2C%22lastName%22%3A%22Schmidt%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kanade%22%2C%22lastName%22%3A%22Shinkai%22%7D%5D%2C%22abstractNote%22%3A%22Hirsutism%2C%20acne%2C%20and%20androgenetic%20alopecia%20are%20classically%20considered%20signs%20of%20cutaneous%20hyperandrogenism%20%28CHA%29.%20These%20common%20skin%20findings%20have%20significant%20impacts%20on%20the%20quality%20of%20patients%26%23039%3B%20lives%20and%20pose%20the%20diagnostic%20challenge%20of%20excluding%20underlying%20disorders.%20Many%20with%20CHA%20have%20normal%20serum%20androgen%20levels.%20Hirsutism%20is%20more%20strongly%20associated%20with%20hyperandrogenism%20than%20are%20acne%20or%20androgenetic%20alopecia.%20Variable%20association%20of%20CHA%20with%20hyperandrogenemia%20results%20from%20the%20complexity%20of%20the%20underlying%20pathophysiology%2C%20including%20factors%20local%20to%20the%20pilosebaceous%20unit.%20CHA%20often%20occurs%20in%20the%20setting%20of%20polycystic%20ovary%20syndrome%2C%20the%20most%20common%20disorder%20of%20hyperandrogenism%2C%20but%20can%20also%20present%20in%20uncommon%20conditions%2C%20including%20nonclassic%20adrenal%20hyperplasia%20and%20androgen-producing%20tumors.%20A%20thorough%20history%20and%20full%20skin%20examination%20are%20important%20to%20guide%20appropriate%20diagnostic%20evaluation.%20Oral%20contraceptive%20pills%20with%20or%20without%20antiandrogens%20can%20provide%20therapeutic%20benefit%20for%20hirsutism%20and%20acne.%20Medical%20options%20for%20androgenetic%20alopecia%20remain%20limited.%20Multidisciplinary%20approaches%20may%20be%20needed%20given%20endocrine%2C%20metabolic%2C%20reproductive%2C%20and%20psychiatric%20disorders%20associated%20with%20CHA.%20More%20high-quality%20studies%20into%20the%20mechanisms%20of%20CHA%20and%20the%20benefits%20of%20antiandrogenic%20therapies%20are%20needed.%20We%20provide%20an%20evidence-based%20review%20of%20key%20diagnostic%20and%20therapeutic%20considerations%20in%20the%20treatment%20of%20women%20with%20CHA.%22%2C%22date%22%3A%222015-10%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.jaad.2015.05.026%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%221097-6787%22%2C%22language%22%3A%22eng%22%2C%22collections%22%3A%5B%2286XZQUNS%22%5D%2C%22dateModified%22%3A%222026-01-18T15%3A29%3A32Z%22%7D%7D%2C%7B%22key%22%3A%22XZ777L7R%22%2C%22library%22%3A%7B%22id%22%3A11711645%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Lizneva%20et%20al.%22%2C%22parsedDate%22%3A%222016-11%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%201.35%3B%20%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%20style%3D%26quot%3Bclear%3A%20left%3B%20%26quot%3B%26gt%3B%5Cn%20%20%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-left-margin%26quot%3B%20style%3D%26quot%3Bfloat%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%26quot%3B%26gt%3B1.%26lt%3B%5C%2Fdiv%26gt%3B%26lt%3Bdiv%20class%3D%26quot%3Bcsl-right-inline%26quot%3B%20style%3D%26quot%3Bmargin%3A%200%20.4em%200%201.5em%3B%26quot%3B%26gt%3BLizneva%20D%2C%20Gavrilova-Jordan%20L%2C%20Walker%20W%2C%20Azziz%20R.%20Androgen%20excess%3A%20Investigations%20and%20management.%20Best%20Pract%20Res%20Clin%20Obstet%20Gynaecol.%202016%20Nov%3B37%3A98%26%23x2013%3B118.%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%20%20%20%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Androgen%20excess%3A%20Investigations%20and%20management%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Daria%22%2C%22lastName%22%3A%22Lizneva%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Larisa%22%2C%22lastName%22%3A%22Gavrilova-Jordan%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Walidah%22%2C%22lastName%22%3A%22Walker%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ricardo%22%2C%22lastName%22%3A%22Azziz%22%7D%5D%2C%22abstractNote%22%3A%22Androgen%20excess%20%28AE%29%20is%20a%20key%20feature%20of%20polycystic%20ovary%20syndrome%20%28PCOS%29%20and%20results%20in%2C%20or%20contributes%20to%2C%20the%20clinical%20phenotype%20of%20these%20patients.%20Although%20AE%20will%20contribute%20to%20the%20ovulatory%20and%20menstrual%20dysfunction%20of%20these%20patients%2C%20the%20most%20recognizable%20sign%20of%20AE%20includes%20hirsutism%2C%20acne%2C%20and%20androgenic%20alopecia%20or%20female%20pattern%20hair%20loss%20%28FPHL%29.%20Evaluation%20includes%20not%20only%20scoring%20facial%20and%20body%20terminal%20hair%20growth%20using%20the%5Cu00a0modified%20Ferriman-Gallwey%20method%20but%20also%20recording%20and%5Cu00a0possibly%20scoring%20acne%20and%20alopecia.%20Moreover%2C%20assessment%20of%5Cu00a0biochemical%20hyperandrogenism%20is%20necessary%2C%20particularly%20in%20patients%20with%20unclear%20or%20absent%20hirsutism%2C%20and%20will%20include%20assessing%20total%20and%20free%20testosterone%20%28T%29%2C%20and%20possibly%20dehydroepiandrosterone%20sulfate%20%28DHEAS%29%20and%20androstenedione%2C%20although%20these%20latter%20contribute%20limitedly%20to%20the%20diagnosis.%20Assessment%20of%20T%5Cu00a0requires%20use%20of%20the%20highest%20quality%20assays%20available%2C%20generally%20radioimmunoassays%20with%20extraction%20and%20chromatography%20or%20mass%20spectrometry%20preceded%20by%20liquid%20or%20gas%20chromatography.%20Management%20of%20clinical%20hyperandrogenism%20involves%20primarily%20either%20androgen%20suppression%2C%20with%20a%20hormonal%20combination%20contraceptive%2C%20or%20androgen%20blockade%2C%20as%20with%20an%20androgen%20receptor%20blocker%20or%20a%205%5Cu03b1-reductase%20inhibitor%2C%20or%20a%20combination%20of%5Cu00a0the%20two.%20Medical%20treatment%20should%20be%20combined%20with%20cosmetic%5Cu00a0treatment%20including%20topical%20eflornithine%20hydrochloride%20and%20short-term%20%28shaving%2C%20chemical%20depilation%2C%20plucking%2C%20threading%2C%20waxing%2C%20and%20bleaching%29%20and%20long-term%20%28electrolysis%2C%20laser%20therapy%2C%20and%20intense%20pulse%20light%20therapy%29%20cosmetic%20treatments.%20Generally%2C%20acne%20responds%20to%20therapy%20relatively%20rapidly%2C%20whereas%20hirsutism%20is%20slower%20to%20respond%2C%20with%20improvements%20observed%20as%20early%20as%203%20months%2C%20but%20routinely%20only%20after%206%20or%208%20months%20of%20therapy.%20Finally%2C%20FPHL%20is%20the%20slowest%20to%20respond%20to%20therapy%2C%20if%20it%20will%20at%20all%2C%20and%20it%20may%20take%2012%20to%2018%20months%20of%20therapy%20for%20an%20observable%20response.%22%2C%22date%22%3A%222016-11%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.bpobgyn.2016.05.003%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%221532-1932%22%2C%22language%22%3A%22eng%22%2C%22collections%22%3A%5B%2286XZQUNS%22%5D%2C%22dateModified%22%3A%222026-01-18T15%3A27%3A01Z%22%7D%7D%2C%7B%22key%22%3A%22QMJKTYY2%22%2C%22library%22%3A%7B%22id%22%3A11711645%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Azziz%22%2C%22parsedDate%22%3A%222018%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%201.35%3B%20%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%20style%3D%26quot%3Bclear%3A%20left%3B%20%26quot%3B%26gt%3B%5Cn%20%20%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-left-margin%26quot%3B%20style%3D%26quot%3Bfloat%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%26quot%3B%26gt%3B1.%26lt%3B%5C%2Fdiv%26gt%3B%26lt%3Bdiv%20class%3D%26quot%3Bcsl-right-inline%26quot%3B%20style%3D%26quot%3Bmargin%3A%200%20.4em%200%201.5em%3B%26quot%3B%26gt%3BAzziz%20R.%20Polycystic%20Ovary%20Syndrome.%20Obstetrics%20%26amp%3B%20Gynecology.%202018%3B132%282%29%3A321%26%23x2013%3B36.%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%20%20%20%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Polycystic%20Ovary%20Syndrome%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ricardo%22%2C%22lastName%22%3A%22Azziz%22%7D%5D%2C%22abstractNote%22%3A%22Polycystic%20ovary%20syndrome%20%28PCOS%29%20is%20a%20highly%20prevalent%20disorder%2C%20representing%20the%20single%20most%20common%20endocrine%5Cu2013metabolic%20disorder%20in%20reproductive-aged%20women.%20Currently%20there%20are%20four%20recognized%20phenotypes%20of%20PCOS%3A%201%29%20hyperandrogenism%2Boligo-anovulation%2Bpolycystic%20ovarian%20morphology%3B%202%29%20hyperandrogenism%2Boligo-anovulation%3B%203%29%20hyperandrogenism%2Bpolycystic%20ovarian%20morphology%3B%20and%204%29%20oligo-anovulation%2Bpolycystic%20ovarian%20morphology%2C%20each%20with%20different%20long-term%20health%20and%20metabolic%20implications.%20Clinicians%20should%20clearly%20denote%20a%20patient%26%23039%3Bs%20phenotype%20when%20making%20the%20diagnosis%20of%20PCOS.%20Polycystic%20ovary%20syndrome%20is%20a%20highly%20inherited%20complex%20polygenic%2C%20multifactorial%20disorder.%20Pathophysiologically%20abnormalities%20in%20gonadotropin%20secretion%20or%20action%2C%20ovarian%20folliculogenesis%2C%20steroidogenesis%2C%20insulin%20secretion%20or%20action%2C%20and%20adipose%20tissue%20function%2C%20among%20others%2C%20have%20been%20described%20in%20PCOS.%20Women%20with%20PCOS%20are%20at%20increased%20risk%20for%20glucose%20intolerance%20and%20type%202%20diabetes%20mellitus%3B%20hepatic%20steatosis%20and%20metabolic%20syndrome%3B%20hypertension%2C%20dyslipidemia%2C%20vascular%20thrombosis%2C%20cerebrovascular%20accidents%2C%20and%20possibly%20cardiovascular%20events%3B%20subfertility%20and%20obstetric%20complications%3B%20endometrial%20atypia%20or%20carcinoma%2C%20and%20possibly%20ovarian%20malignancy%3B%20and%20mood%20and%20psychosexual%20disorders.%20The%20evaluation%20of%20patients%20suspected%20of%20having%20PCOS%20includes%20a%20thorough%20history%20and%20physical%20examination%2C%20assessment%20for%20the%20presence%20of%20hirsutism%2C%20ovarian%20ultrasonography%2C%20and%20hormonal%20testing%20to%20confirm%20hyperandrogenism%20and%20oligo-anovulation%20as%20needed%20and%20to%20exclude%20similar%20or%20mimicking%20disorders.%20Therapeutic%20decisions%20in%20PCOS%20depend%20on%20the%20patients%26%23039%3B%20phenotype%2C%20concerns%2C%20and%20goals%2C%20and%20should%20focus%20on%201%29%20suppressing%20and%20counteracting%20androgen%20secretion%20and%20action%2C%202%29%20improving%20metabolic%20status%2C%20and%203%29%20improving%20fertility.%20However%2C%20despite%20significant%20progress%20in%20understanding%20the%20pathophysiology%20and%20diagnosis%20of%20the%20disorder%20over%20the%20past%2020%20years%2C%20the%20disorder%20remains%20underdiagnosed%20and%20misunderstood%20by%20many%20practitioners.%22%2C%22date%22%3A%2208%5C%2F2018%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%2210.1097%5C%2FAOG.0000000000002698%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%220029-7844%22%2C%22language%22%3A%22en%22%2C%22collections%22%3A%5B%2286XZQUNS%22%5D%2C%22dateModified%22%3A%222026-01-18T15%3A25%3A12Z%22%7D%7D%2C%7B%22key%22%3A%22X8DIRN4F%22%2C%22library%22%3A%7B%22id%22%3A11711645%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Carmina%20et%20al.%22%2C%22parsedDate%22%3A%222019-07-01%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%201.35%3B%20%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%20style%3D%26quot%3Bclear%3A%20left%3B%20%26quot%3B%26gt%3B%5Cn%20%20%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-left-margin%26quot%3B%20style%3D%26quot%3Bfloat%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%26quot%3B%26gt%3B1.%26lt%3B%5C%2Fdiv%26gt%3B%26lt%3Bdiv%20class%3D%26quot%3Bcsl-right-inline%26quot%3B%20style%3D%26quot%3Bmargin%3A%200%20.4em%200%201.5em%3B%26quot%3B%26gt%3BCarmina%20E%2C%20Azziz%20R%2C%20Bergfeld%20W%2C%20Escobar-Morreale%20HF%2C%20Futterweit%20W%2C%20Huddleston%20H%2C%20et%20al.%20Female%20Pattern%20Hair%20Loss%20and%20Androgen%20Excess%3A%20A%20Report%20From%20the%20Multidisciplinary%20Androgen%20Excess%20and%20PCOS%20Committee.%20J%20Clin%20Endocrinol%20Metab.%202019%20July%201%3B104%287%29%3A2875%26%23x2013%3B91.%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%20%20%20%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Female%20Pattern%20Hair%20Loss%20and%20Androgen%20Excess%3A%20A%20Report%20From%20the%20Multidisciplinary%20Androgen%20Excess%20and%20PCOS%20Committee%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Enrico%22%2C%22lastName%22%3A%22Carmina%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ricardo%22%2C%22lastName%22%3A%22Azziz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Wilma%22%2C%22lastName%22%3A%22Bergfeld%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22H%5Cu00e9ctor%20F.%22%2C%22lastName%22%3A%22Escobar-Morreale%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Walter%22%2C%22lastName%22%3A%22Futterweit%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Heather%22%2C%22lastName%22%3A%22Huddleston%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Rogerio%22%2C%22lastName%22%3A%22Lobo%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Elise%22%2C%22lastName%22%3A%22Olsen%22%7D%5D%2C%22abstractNote%22%3A%22OBJECTIVE%3A%20To%20determine%20the%20current%20state%20of%20knowledge%20and%20provide%20evidence-based%20recommendations%20that%20could%20be%20valid%20for%20all%20specialists%20taking%20care%20of%20female%20pattern%20hair%20loss%20%28FPHL%29%2C%20a%20common%20form%20of%20hair%20loss%20in%20women%20that%20is%20characterized%20by%20the%20reduction%20of%20hair%20density%20in%20the%20central%20area%20of%20the%20scalp%2C%20whereas%20the%20frontal%20hairline%20is%20generally%20well%20conserved.%5CnPARTICIPANTS%3A%20An%20expert%20task%20force%20appointed%20by%20the%20Androgen%20Excess%20and%20PCOS%20Society%2C%20which%20included%20specialists%20from%20dermatology%2C%20endocrinology%2C%20and%20reproductive%20endocrinology.%5CnDESIGN%3A%20Levels%20of%20evidence%20were%20assessed%20and%20graded%20from%20A%20to%20D.%20Peer-reviewed%20studies%20evaluating%20FPHL%20published%20through%20December%202017%20were%20reviewed.%20Criteria%20for%20inclusion%5C%2Fexclusion%20of%20the%20published%20papers%20were%20agreed%20on%20by%20at%20least%20two%20reviewers%20in%20each%20area%20and%20arbitrated%20by%20a%20third%20when%20necessary.%5CnCONCLUSIONS%3A%20%28i%29%20The%20term%20%26quot%3Bfemale%20pattern%20hair%20loss%26quot%3B%20should%20be%20used%2C%20avoiding%20the%20previous%20terms%20of%20alopecia%20or%20androgenetic%20alopecia.%20%28ii%29%20The%20two%20typical%20patterns%20of%20hair%20loss%20in%20FPHL%20are%20centrifugal%20expansion%20in%20the%20mid%20scalp%2C%20and%20a%20frontal%20accentuation%20or%20Christmas%20tree%20pattern.%20%28iii%29%20Isolated%20FPHL%20should%20not%20be%20considered%20a%20sign%20of%20hyperandrogenism%20when%20androgen%20levels%20are%20normal.%20%28iv%29%20The%20assessment%20of%20patients%20with%20FPHL%20is%20primarily%20clinical.%20%28v%29%20In%20all%20patients%20with%20FPHL%2C%20assessment%20of%20a%20possible%20androgen%20excess%20is%20mandatory.%20Measurement%20of%20vitamin%20D%2C%20iron%2C%20zinc%2C%20thyroid%20hormones%2C%20and%20prolactin%20are%20optional%20but%20recommended.%20%28vi%29%20Treatment%20of%20FPHL%20should%20start%20with%20minoxidil%20%285%25%29%2C%20adding%205%5Cu03b1-reductase%20inhibitors%20or%20antiandrogens%20when%20there%20is%20severe%20hair%20loss%20or%20hyperandrogenism.%22%2C%22date%22%3A%222019-07-01%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%2210.1210%5C%2Fjc.2018-02548%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%221945-7197%22%2C%22language%22%3A%22eng%22%2C%22collections%22%3A%5B%2286XZQUNS%22%5D%2C%22dateModified%22%3A%222026-01-18T15%3A25%3A58Z%22%7D%7D%2C%7B%22key%22%3A%22QL9K7MKK%22%2C%22library%22%3A%7B%22id%22%3A11711645%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Jiang%20et%20al.%22%2C%22parsedDate%22%3A%222022-12-01%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%201.35%3B%20%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%20style%3D%26quot%3Bclear%3A%20left%3B%20%26quot%3B%26gt%3B%5Cn%20%20%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-left-margin%26quot%3B%20style%3D%26quot%3Bfloat%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%26quot%3B%26gt%3B1.%26lt%3B%5C%2Fdiv%26gt%3B%26lt%3Bdiv%20class%3D%26quot%3Bcsl-right-inline%26quot%3B%20style%3D%26quot%3Bmargin%3A%200%20.4em%200%201.5em%3B%26quot%3B%26gt%3BJiang%20VS%2C%20Hawkins%20SD%2C%20McMichael%20A.%20Female%20pattern%20hair%20loss%20and%20polycystic%20ovarian%20syndrome%3A%20more%20than%20just%20hirsutism.%20Curr%20Opin%20Endocrinol%20Diabetes%20Obes.%202022%20Dec%201%3B29%286%29%3A535%26%23x2013%3B40.%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%20%20%20%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Female%20pattern%20hair%20loss%20and%20polycystic%20ovarian%20syndrome%3A%20more%20than%20just%20hirsutism%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Victoria%20S.%22%2C%22lastName%22%3A%22Jiang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Spencer%20D.%22%2C%22lastName%22%3A%22Hawkins%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Amy%22%2C%22lastName%22%3A%22McMichael%22%7D%5D%2C%22abstractNote%22%3A%22PURPOSE%20OF%20REVIEW%3A%20To%20explore%20the%20recent%20updates%20in%20the%20diagnosis%2C%20management%2C%20and%20clinical%20implications%20of%20androgenic%20alopecia%20among%20patients%20diagnosed%20with%20polycystic%20ovarian%20syndrome%20%28PCOS%29.%5CnRECENT%20FINDINGS%3A%20PCOS%20diagnosis%20continues%20to%20be%20the%20most%20common%20cause%20of%20infertility%20among%20reproductively%20aged%20women%2C%20serving%20as%20the%20most%20common%20endocrinopathy%20among%20this%20population.%20Female%20pattern%20hair%20loss%20%28FPHL%29%20has%20been%20seen%20to%20be%20associated%20and%20more%20common%20among%20patients%20with%20PCOS%2C%20however%2C%20there%20are%20limited%20studies%20examining%20the%20impact%20of%20FPHL%20among%20PCOS%20patients.%20Although%20hyperandrogenism%20is%20associated%20with%20FPHL%2C%20the%20pathophysiology%20continues%20to%20be%20unclear%20as%20FPHL%20can%20be%20present%20with%20normal%20biochemical%20androgen%20markers.%20Treatment%20can%20be%20complex%2C%20as%20common%20treatments%20to%20promote%20hair%20growth%20can%20exacerbate%20undesired%20hirsutism%2C%20which%20can%20be%20overcome%20by%20cosmetic%20treatments.%20New%20second-line%20treatment%20options%20such%20as%20low%20level%20laser%20therapy%20and%20platelet%20rich%20plasma%20have%20been%20emerging%2C%20with%20limited%20data%20supporting%20efficacy.%5CnSUMMARY%3A%20PCOS%20is%20a%20complex%20endocrinological%20disorder%20that%20has%20significant%20gynecologic%2C%20cutaneous%2C%20and%20metabolic%20implications%20that%20require%20multidisciplinary%20collaboration%20and%20care.%20Reproductive%20goals%20should%20be%20thoroughly%20discussed%20prior%20to%20starting%20any%20treatment%2C%20as%20PCOS%20is%20the%20most%20common%20cause%20of%20infertility%20among%20reproductively-aged%20women.%22%2C%22date%22%3A%222022-12-01%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%2210.1097%5C%2FMED.0000000000000777%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%221752-2978%22%2C%22language%22%3A%22eng%22%2C%22collections%22%3A%5B%2286XZQUNS%22%5D%2C%22dateModified%22%3A%222026-01-18T15%3A29%3A05Z%22%7D%7D%2C%7B%22key%22%3A%22PURXBVQY%22%2C%22library%22%3A%7B%22id%22%3A11711645%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Klein%20et%20al.%22%2C%22parsedDate%22%3A%222023-01-06%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%201.35%3B%20%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%20style%3D%26quot%3Bclear%3A%20left%3B%20%26quot%3B%26gt%3B%5Cn%20%20%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-left-margin%26quot%3B%20style%3D%26quot%3Bfloat%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%26quot%3B%26gt%3B1.%26lt%3B%5C%2Fdiv%26gt%3B%26lt%3Bdiv%20class%3D%26quot%3Bcsl-right-inline%26quot%3B%20style%3D%26quot%3Bmargin%3A%200%20.4em%200%201.5em%3B%26quot%3B%26gt%3BKlein%20EJ%2C%20Oh%20CS%2C%20Karim%20M%2C%20Shapiro%20J%2C%20Lo%20Sicco%20K.%20A%20practical%20approach%20to%20the%20management%20of%20hair%20loss%20in%20patients%20with%20polycystic%20ovary%20syndrome.%20J%20Eur%20Acad%20Dermatol%20Venereol.%202023%20Jan%206%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%20%20%20%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22A%20practical%20approach%20to%20the%20management%20of%20hair%20loss%20in%20patients%20with%20polycystic%20ovary%20syndrome%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Elizabeth%20J.%22%2C%22lastName%22%3A%22Klein%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Christina%20S.%22%2C%22lastName%22%3A%22Oh%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Maria%22%2C%22lastName%22%3A%22Karim%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jerry%22%2C%22lastName%22%3A%22Shapiro%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kristen%22%2C%22lastName%22%3A%22Lo%20Sicco%22%7D%5D%2C%22abstractNote%22%3A%22Female%20patterned%20hair%20loss%20%28FPHL%29%20is%20a%20common%20form%20of%20androgenetic%20alopecia%20in%20women%20and%20is%20characterized%20by%20a%20hormonally%20directed%20diffuse%20hair%20loss%20on%20the%20scalp.%20Management%20of%20FPHL%20is%20well%20described%20in%20the%20literature%3B%20however%2C%20treatment%20of%20FPHL%20in%20patients%20with%20co-morbid%20polycystic%20ovarian%20syndrome%20%28PCOS%29%2C%20an%20endocrinologic%20condition%20found%20in%20reproductive-aged%20women%2C%20has%20not%20yet%20been%20reviewed.%20Due%20to%20the%20different%20pathomechanism%20of%20the%20diseases%20and%20complexity%20of%20FPHL%20in%20PCOS%20patients%2C%20this%20study%20aimed%20to%20review%20current%20diagnosis%20and%20management%20approaches%20for%20hair%20loss%20in%20PCOS%20patients%20specifically%20and%20highlight%20the%20growing%20need%20for%20more%20research%20in%20this%20growing%20patient%20population.%22%2C%22date%22%3A%222023-01-06%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%2210.1111%5C%2Fjdv.18842%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%221468-3083%22%2C%22language%22%3A%22eng%22%2C%22collections%22%3A%5B%2286XZQUNS%22%5D%2C%22dateModified%22%3A%222026-01-18T15%3A24%3A07Z%22%7D%7D%2C%7B%22key%22%3A%2257WEALKF%22%2C%22library%22%3A%7B%22id%22%3A11711645%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Desai%20et%20al.%22%2C%22parsedDate%22%3A%222024-09-14%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%201.35%3B%20%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%20style%3D%26quot%3Bclear%3A%20left%3B%20%26quot%3B%26gt%3B%5Cn%20%20%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-left-margin%26quot%3B%20style%3D%26quot%3Bfloat%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%26quot%3B%26gt%3B1.%26lt%3B%5C%2Fdiv%26gt%3B%26lt%3Bdiv%20class%3D%26quot%3Bcsl-right-inline%26quot%3B%20style%3D%26quot%3Bmargin%3A%200%20.4em%200%201.5em%3B%26quot%3B%26gt%3BDesai%20DD%2C%20Nohria%20A%2C%20Sikora%20M%2C%20Anyanwu%20N%2C%20Shapiro%20J%2C%20Lo%20Sicco%20KI.%20Comparative%20analysis%20of%20low-dose%20oral%20minoxidil%20with%20spironolactone%20versus%20finasteride%20or%20dutasteride%20in%20female%20androgenetic%20alopecia%20management.%20Arch%20Dermatol%20Res.%202024%20Sept%2014%3B316%289%29%3A622.%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%20%20%20%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Comparative%20analysis%20of%20low-dose%20oral%20minoxidil%20with%20spironolactone%20versus%20finasteride%20or%20dutasteride%20in%20female%20androgenetic%20alopecia%20management%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Deesha%20D.%22%2C%22lastName%22%3A%22Desai%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ambika%22%2C%22lastName%22%3A%22Nohria%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Michelle%22%2C%22lastName%22%3A%22Sikora%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nnaemeka%22%2C%22lastName%22%3A%22Anyanwu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jerry%22%2C%22lastName%22%3A%22Shapiro%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kristen%20I.%22%2C%22lastName%22%3A%22Lo%20Sicco%22%7D%5D%2C%22abstractNote%22%3A%22LDOM%20has%20enhanced%20treatment%20options%20for%20female%20AGA%2C%20yet%20its%20combined%20efficacy%20with%20therapies%20such%20as%20spironolactone%2C%20finasteride%2C%20or%20dutasteride%20remains%20inadequately%20explored.%20This%20study%20aims%20to%20compare%20the%20efficacy%20and%20safety%20of%20LDOM%20in%20combination%20with%20spironolactone%20versus%20LDOM%20with%20finasteride%20or%20dutasteride%20in%20women%20with%20AGA.%20Our%20analysis%20revealed%20that%20both%20combination%20therapies%20produced%20similar%20improvements%20in%20hair%20growth%20and%20had%20comparable%20safety%20profiles.%20Although%20the%20LDOM%20with%20finasteride%5C%2Fdutasteride%20group%20showed%20a%20greater%20average%20increase%20in%20hair%20width%20and%20density%2C%20these%20differences%20were%20not%20statistically%20significant.%20These%20results%20endorse%20the%20use%20of%20LDOM%20in%20combination%20with%20either%20spironolactone%20or%20finasteride%5C%2Fdutasteride%20for%20female%20AGA%2C%20and%20underscore%20the%20necessity%20for%20further%20research%20to%20validate%20these%20findings%20and%20assess%20long-term%20treatment%20outcomes.%22%2C%22date%22%3A%222024-09-14%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%2210.1007%5C%2Fs00403-024-03361-x%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%221432-069X%22%2C%22language%22%3A%22eng%22%2C%22collections%22%3A%5B%2286XZQUNS%22%5D%2C%22dateModified%22%3A%222026-01-18T15%3A32%3A09Z%22%7D%7D%2C%7B%22key%22%3A%226QJLUINJ%22%2C%22library%22%3A%7B%22id%22%3A11711645%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Werachattawatchai%20et%20al.%22%2C%22parsedDate%22%3A%222025-10%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%201.35%3B%20%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%20style%3D%26quot%3Bclear%3A%20left%3B%20%26quot%3B%26gt%3B%5Cn%20%20%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-left-margin%26quot%3B%20style%3D%26quot%3Bfloat%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%26quot%3B%26gt%3B1.%26lt%3B%5C%2Fdiv%26gt%3B%26lt%3Bdiv%20class%3D%26quot%3Bcsl-right-inline%26quot%3B%20style%3D%26quot%3Bmargin%3A%200%20.4em%200%201.5em%3B%26quot%3B%26gt%3BWerachattawatchai%20P%2C%20Khunkhet%20S%2C%20Harnchoowong%20S%2C%20Lertphanichkul%20C.%20Efficacy%20and%20safety%20of%20oral%20spironolactone%20for%20female%20pattern%20hair%20loss%20in%20premenopausal%20women%3A%20a%20randomized%2C%20double-blind%2C%20placebo-controlled%2C%20parallel-group%20pilot%20study.%20Int%20J%20Womens%20Dermatol.%202025%20Oct%3B11%283%29%3Ae227.%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%20%20%20%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Efficacy%20and%20safety%20of%20oral%20spironolactone%20for%20female%20pattern%20hair%20loss%20in%20premenopausal%20women%3A%20a%20randomized%2C%20double-blind%2C%20placebo-controlled%2C%20parallel-group%20pilot%20study%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Pimpakarn%22%2C%22lastName%22%3A%22Werachattawatchai%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Saranya%22%2C%22lastName%22%3A%22Khunkhet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sarawin%22%2C%22lastName%22%3A%22Harnchoowong%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Chotinij%22%2C%22lastName%22%3A%22Lertphanichkul%22%7D%5D%2C%22abstractNote%22%3A%22BACKGROUND%3A%20Oral%20spironolactone%20is%20widely%20used%20as%20an%20alternative%20treatment%20for%20female%20pattern%20hair%20loss%2C%20particularly%20in%20women%20of%20reproductive%20age.%20However%2C%20published%20data%20on%20its%20effects%20are%20still%20limited.%5CnOBJECTIVE%3A%20This%20study%20aimed%20to%20evaluate%20the%20efficacy%20and%20safety%20of%20spironolactone%20100%5Cu2009mg%20daily%20for%20female%20pattern%20hair%20loss%20in%20premenopausal%20women%20compared%20with%20placebo.%5CnMETHODS%3A%20Forty-eight%20women%20aged%2021%20to%2045%20years%20were%20randomly%20assigned%20to%20receive%20spironolactone%20100%5Cu2009mg%20or%20matched%20placebo%20once%20daily%20for%2024%20weeks.%20Twice-daily%20minoxidil%203%25%20solution%20was%20used%20for%20all%20to%20avoid%20untreated%20participants.%20Efficacy%20was%20assessed%20by%20changes%20in%20hair%20density%20and%20diameter%20using%20videodermoscopy%20and%20global%20photographic%20assessment.%5CnRESULTS%3A%20After%20treatment%2C%20hair%20density%20and%20diameter%20increased%20significantly%20in%20both%20treatment%20groups%20%28all%20P%20%26lt%3B%200.001%29.%20The%20spironolactone%20group%20showed%20a%20greater%20increase%20in%20changes%20from%20baseline%20of%20terminal%20hair%20counts%20%289.48%5Cu2009%5Cu00b1%5Cu200911.25%20vs%205.32%5Cu2009%5Cu00b1%5Cu20096.81%20hairs%5C%2Fcm2%29%20and%20hair%20diameters%20%284.23%5Cu2009%5Cu00b1%5Cu20094.58%20vs%202.96%5Cu2009%5Cu00b1%5Cu20092.84%20%5Cu03bcm%29%2C%20compared%20with%20the%20placebo%20group.%20However%2C%20only%20the%20P-value%20of%20terminal%20hair%20counts%20approached%20the%20cutoff%20of%20statistical%20significance%20%28P%20%3D%20.063%29.%20The%20spironolactone%20group%20also%20showed%20greater%20moderate-to-marked%20improvement%20than%20the%20placebo%20group%20%2838%25%20vs%209%25%3B%20P%20%3D%20.034%29.%20Adverse%20events%2C%20primarily%20menstrual%20irregularities%20%2837.5%25%29%2C%20were%20significantly%20higher%20in%20the%20spironolactone%20group.%5CnLIMITATIONS%3A%20The%20sample%20size%20was%20small.%5CnCONCLUSION%3A%20Oral%20spironolactone%20100%5Cu2009mg%20daily%20for%2024%20weeks%20showed%20an%20additive%20effect%20on%20topical%20minoxidil%20therapy%20in%20premenopausal%20women%20with%20mild-to-moderate%20female%20pattern%20hair%20loss.%20Although%20it%20was%20generally%20well-tolerated%2C%20irregular%20menstruation%20was%20common.%22%2C%22date%22%3A%222025-10%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%2210.1097%5C%2FJW9.0000000000000227%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%222352-6475%22%2C%22language%22%3A%22eng%22%2C%22collections%22%3A%5B%2286XZQUNS%22%5D%2C%22dateModified%22%3A%222026-01-18T15%3A32%3A48Z%22%7D%7D%5D%7D 1.
Sinclair R, Wewerinke M, Jolley D. Treatment of female pattern hair loss with oral antiandrogens. Br J Dermatol. 2005 Mar;152(3):466–73.
1.
Hoedemaker C, van Egmond S, Sinclair R. Treatment of female pattern hair loss with a combination of spironolactone and minoxidil. Australas J Dermatol. 2007 Feb;48(1):43–5.
1.
Rathnayake D, Sinclair R. Innovative use of spironolactone as an antiandrogen in the treatment of female pattern hair loss. Dermatol Clin. 2010 July;28(3):611–8.
1.
Schmidt TH, Shinkai K. Evidence-based approach to cutaneous hyperandrogenism in women. J Am Acad Dermatol. 2015 Oct;73(4):672–90.
1.
Lizneva D, Gavrilova-Jordan L, Walker W, Azziz R. Androgen excess: Investigations and management. Best Pract Res Clin Obstet Gynaecol. 2016 Nov;37:98–118.
1.
Azziz R. Polycystic Ovary Syndrome. Obstetrics & Gynecology. 2018;132(2):321–36.
1.
Carmina E, Azziz R, Bergfeld W, Escobar-Morreale HF, Futterweit W, Huddleston H, et al. Female Pattern Hair Loss and Androgen Excess: A Report From the Multidisciplinary Androgen Excess and PCOS Committee. J Clin Endocrinol Metab. 2019 July 1;104(7):2875–91.
1.
Jiang VS, Hawkins SD, McMichael A. Female pattern hair loss and polycystic ovarian syndrome: more than just hirsutism. Curr Opin Endocrinol Diabetes Obes. 2022 Dec 1;29(6):535–40.
1.
Klein EJ, Oh CS, Karim M, Shapiro J, Lo Sicco K. A practical approach to the management of hair loss in patients with polycystic ovary syndrome. J Eur Acad Dermatol Venereol. 2023 Jan 6;
1.
Desai DD, Nohria A, Sikora M, Anyanwu N, Shapiro J, Lo Sicco KI. Comparative analysis of low-dose oral minoxidil with spironolactone versus finasteride or dutasteride in female androgenetic alopecia management. Arch Dermatol Res. 2024 Sept 14;316(9):622.
1.
Werachattawatchai P, Khunkhet S, Harnchoowong S, Lertphanichkul C. Efficacy and safety of oral spironolactone for female pattern hair loss in premenopausal women: a randomized, double-blind, placebo-controlled, parallel-group pilot study. Int J Womens Dermatol. 2025 Oct;11(3):e227.