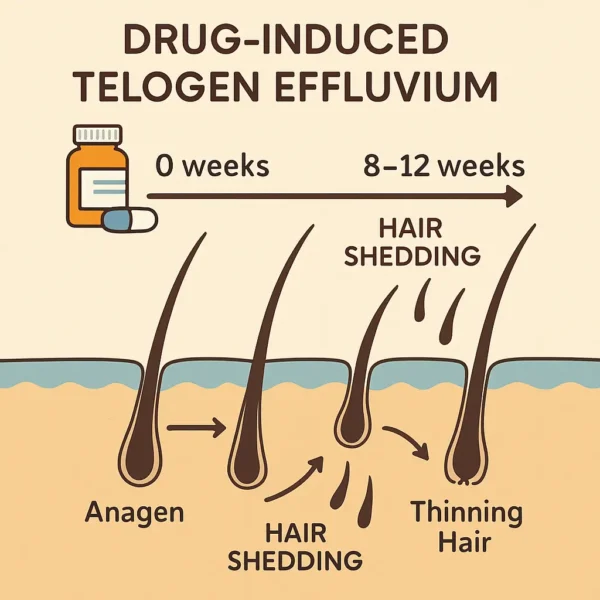
Understanding Telogen Effluvium: Telogen effluvium (TE) is a nonscarring , reversible form of diffuse hair loss characterized by an increased proportion of hair follicles entering the resting (telogen) phase and subsequent shedding. Under physiologic conditions, roughly 5–15 % of scalp hairs are in telogen; in TE, a synchronized shift causes many more hairs to stop growing and detach several weeks later, leading to noticeable thinning across the entire scalp rather than discrete bald patches. Unlike anagen effluvium (rapid loss of growing hairs, typically within days of insult), TE unfolds over 1–6 months after a trigger and primarily affects hairs in their resting phase.
How Medications Trigger Hair Shedding: Medications can provoke telogen effluvium by inducing “immediate anagen release”, wherein hair follicles prematurely exit the anagen growth phase. Key mechanisms include:
Disruption of growth signaling. Many drugs can interfere with follicular growth factors or hormone signals that control the hair growth cycle and particularly those that maintain the anagen hair growth phase. The result is a mass conversion of follicles into telogen, with shedding delayed by the length of the telogen rest phase – usually 2–4 months later.Micronutrient depletion. Agents, such as valproic acid for example, can chelate zinc and selenium or reduce biotin recycling, depriving follicles of essential nutrients for keratin synthesis and pushing them into telogen (“nutritional TE”).Molecular signaling changes. Systemic retinoids (e.g., isotretinoin, acitretin) alter gene expression in follicular keratinocytes – upregulating pathways (e.g., TGF-β/SMAD) that trigger catagen and telogen, with severity often correlating to dose and duration of the drug exposure.Circulatory or inflammatory effects. Some cardiovascular drugs (beta-blockers, anticoagulants) may subtly reduce scalp perfusion or alter local cytokines, stressing follicles into telogen. Immune-modulating agents can also provoke mild inflammation that secondarily affects the cycle. While these mechanisms vary by drug, the unifying feature is a delayed, diffuse shedding of telogen hairs from follicles previously in anagen when the drug begins to exert its effect. Because of the hair cycle’s latency, patients often do not notice increased hair loss until some months after starting therapy, making the causal link less obvious.
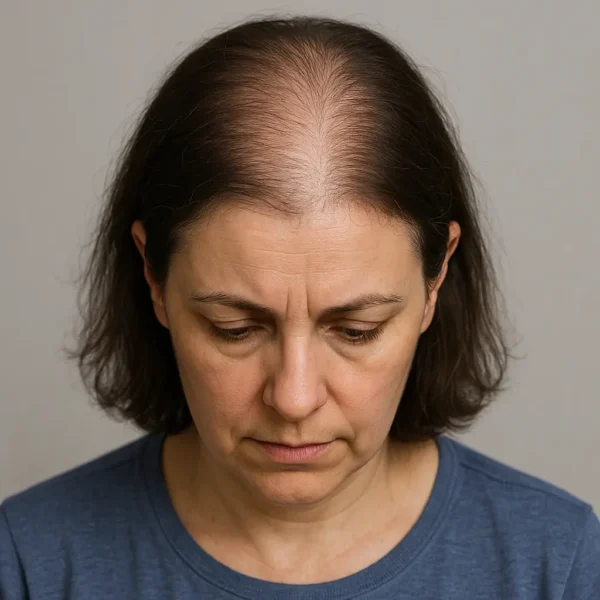
Clinical Presentation : There is typically no obvious pattern to telogen effluvium, but there tends to several features that are usually (though not always) present:
Diffuse thinning across the scalp, most visible at the vertex but present everywhere.Increased daily shedding , often exceeding 100 hairs/day, with hairs having small “club” bulbs at their roots.Normal scalp skin , lacking scarring, inflammation, or scaling.Preserved frontal hairline , differentiating TE from pattern hair loss.Mild eyebrow or body hair thinning can occasionally occur, but scalp involvement dominates because of its high anagen fraction and longer hair growth cycle length. The delay between drug initiation and hair loss (commonly 8–12 weeks) is a diagnostic hallmark. Patients may describe seeing clumps of hair on pillows or in the shower well after they began a new medication, leading to delayed recognition of the association.
Medications Most Commonly Implicated: Although any systemic drug stressing the hair cycle can cause TE, the following classes and examples are most often reported:
1. Antidepressants:
SSRIs (sertraline, fluoxetine, paroxetine, citalopram): Case reports and systematic reviews document alopecia in <1 % of users, typically onset at ~8–9 weeks of therapy.Bupropion : Larger cohort studies suggest ~1 % of patients experience TE, a rate somewhat higher than SSRIs.Tricyclics and other agents (amitriptyline, imipramine) have also been linked, albeit infrequently. 2. Anticonvulsants and Mood Stabilizers:
Valproic acid (valproate, divalproex): Reported hair loss in 10–11 % of users, dose-dependent, driven by nutrient depletion and possibly direct follicle effects.Carbamazepine : TE in ~4–6 % of patients.Lamotrigine and other antiseizure drugs : Sporadic reports of diffuse shedding months into treatment. 3. Retinoids (Vitamin A Derivatives):
Isotretinoin (high-dose acne therapy): Alopecia is seen in ~3–6 % of patients, reversible upon cessation of the drug.Acitretin (psoriasis): High-dose regimens (>50 mg/day) can cause TE in over 60% of cases, with dose and duration correlating with severity. 4. Cardiovascular and Hematologic Agents:
Beta-blockers (propranolol, metoprolol): Rare cases of diffuse TE attributed to altered scalp perfusion or hormonal changes.Anticoagulants (warfarin, heparin and newer direct oral anticoagulants): Known to induce TE in susceptible individuals, often 3–4 months after initiation. 5. Other Notable Culprits:
Systemic azole antifungals (fluconazole, voriconazole): High-dose courses can precipitate TE around 3 months into therapy.Hormone modulators (oral contraceptives, thyroid hormone therapies): Initiation or discontinuation can unbalance estrogen‐androgen ratios or thyroid levels, triggering TE.Targeted cancer therapies (some tyrosine kinase inhibitors): Cases of gradual, TE-like hair thinning reported, distinct from the acute anagen effluvium hair loss of classical chemotherapy. While the absolute risk for any given individual is low, clinicians should maintain a high index of suspicion when patients on these medications present with diffuse hair loss several weeks to months after starting therapy.
Diagnosis – Timing Is Everything: Diagnosing drug-induced TE relies on:
Detailed medication history: Document all new drugs or dose changes in the past 6 months.Clinical examination: Confirm diffuse thinning, normal scalp skin, and presence of telogen hairs on pull test.Exclusion of other causes: Rule out systemic illnesses, nutritional deficiencies, thyroid dysfunction, and significant stressors.Temporal correlation: A delay of 2–4 months between drug initiation and shedding aligns with drug induced TE kinetics. Because hair follicles remain alive during TE, prognosis is excellent. Counseling patients on the reversible nature of TE can alleviate anxiety and support adherence to critical medications when cessation is not feasible. Some patients panic when they see hair loss and decide to come off the drugs, but of course this is rather dangerous considering the drugs have likely been prescribed for significant health conditions. Consultation with doctors may lead to a reduction in drug dosage or a switch to a similar drug with a slightly different mode of action, often with good results.
Conclusion: Drug-induced telogen effluvium exemplifies the sensitivity of hair follicles to internal perturbations. Although developing diffuse hair thinning months into therapy can be distressing, recognizing the characteristic timing and pattern of TE is crucial for accurate diagnosis. A thorough drug history often reveals the culprit, and in nearly all cases, hair regrowth ensues once the medication is adjusted or replaced with an alternative. Awareness of this condition across diverse drug classes – including antidepressants, anticonvulsants, retinoids, cardiovascular agents, and more – empowers both clinicians and patients to manage hair loss effectively while maintaining necessary medical therapies.
Bibliography
11711645 {11711645:FFUWSSN7},{11711645:2H98I8KA},{11711645:E3X7DJWT},{11711645:8U6EXSJE},{11711645:8QV6WHNT},{11711645:9SEFZ9ED},{11711645:EF2DDBYK},{11711645:VIHUHB8H},{11711645:6CE3TUXQ},{11711645:JLSI5HU6},{11711645:AJ59ZGVS} 1 vancouver 50 date asc 1815 https://www.keratin.com/wp-content/plugins/zotpress/ %7B%22status%22%3A%22success%22%2C%22updateneeded%22%3Afalse%2C%22instance%22%3Afalse%2C%22meta%22%3A%7B%22request_last%22%3A0%2C%22request_next%22%3A0%2C%22used_cache%22%3Atrue%7D%2C%22data%22%3A%5B%7B%22key%22%3A%22FFUWSSN7%22%2C%22library%22%3A%7B%22id%22%3A11711645%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Tosi%20et%20al.%22%2C%22parsedDate%22%3A%221994-04%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%201.35%3B%20%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%20style%3D%26quot%3Bclear%3A%20left%3B%20%26quot%3B%26gt%3B%5Cn%20%20%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-left-margin%26quot%3B%20style%3D%26quot%3Bfloat%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%26quot%3B%26gt%3B1.%26lt%3B%5C%2Fdiv%26gt%3B%26lt%3Bdiv%20class%3D%26quot%3Bcsl-right-inline%26quot%3B%20style%3D%26quot%3Bmargin%3A%200%20.4em%200%201.5em%3B%26quot%3B%26gt%3BTosi%20A%2C%20Misciali%20C%2C%20Piraccini%20BM%2C%20Peluso%20AM%2C%20Bardazzi%20F.%20Drug-induced%20hair%20loss%20and%20hair%20growth.%20Incidence%2C%20management%20and%20avoidance.%20Drug%20Saf.%201994%20Apr%3B10%284%29%3A310%26%23x2013%3B7.%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%20%20%20%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Drug-induced%20hair%20loss%20and%20hair%20growth.%20Incidence%2C%20management%20and%20avoidance%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22A.%22%2C%22lastName%22%3A%22Tosi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22C.%22%2C%22lastName%22%3A%22Misciali%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22B.%20M.%22%2C%22lastName%22%3A%22Piraccini%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22A.%20M.%22%2C%22lastName%22%3A%22Peluso%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22F.%22%2C%22lastName%22%3A%22Bardazzi%22%7D%5D%2C%22abstractNote%22%3A%22A%20large%20number%20of%20drugs%20may%20interfere%20with%20the%20hair%20cycle%20and%20produce%20hair%20loss.%20Drugs%20may%20affect%20anagen%20follicles%20through%202%20main%20different%20modalities%3A%20%28i%29%20by%20inducing%20an%20abrupt%20cessation%20of%20mitotic%20activity%20in%20rapidly%20dividing%20hair%20matrix%20cells%20%28anagen%20effluvium%29%20or%20%28ii%29%20by%20precipitating%20the%20follicles%20into%20premature%20rest%20%28telogen%20effluvium%29.%20In%20anagen%20effluvium%2C%20hair%20loss%20usually%20occurs%20within%20days%20to%20weeks%20of%20drug%20administration%2C%20whereas%20in%20telogen%20effluvium%2C%20hair%20loss%20becomes%20evident%202%20to%204%20months%20after%20starting%20treatment.%20Anagen%20effluvium%20is%20a%20prominent%20adverse%20effect%20of%20antineoplastic%20agents%2C%20which%20cause%20acute%20damage%20of%20rapidly%20dividing%20hair%20matrix%20cells.%20Telogen%20effluvium%20may%20be%20a%20consequence%20of%20a%20large%20number%20of%20drugs%20including%20anticoagulants%2C%20retinol%20%28vitamin%20A%29%20and%20its%20derivatives%2C%20interferons%20and%20antihyperlipidaemic%20drugs.%20Drug-induced%20hair%20loss%20is%20usually%20reversible%20after%20interruption%20of%20treatment.%20The%20prevalence%20and%20severity%20of%20alopecia%20depend%20on%20the%20drug%20as%20well%20as%20on%20individual%20predisposition.%20Some%20drugs%20produce%20hair%20loss%20in%20most%20patients%20receiving%20appropriate%20dosages%20while%20other%20drugs%20are%20only%20occasionally%20responsible%20for%20hair%20abnormalities.%20Both%20hirsutism%20and%20hypertrichosis%20may%20be%20associated%20with%20drug%20administration.%20Drugs%20most%20commonly%20responsible%20for%20the%20development%20of%20hirsutism%20include%20testosterone%2C%20danazol%2C%20corticotrophin%20%28ACTH%29%2C%20metyrapone%2C%20anabolic%20steroids%20and%20glucocorticoids.%20Hypertrichosis%20is%20a%20common%20adverse%20effect%20of%20cyclosporin%2C%20minoxidil%20and%20diazoxide.%22%2C%22date%22%3A%221994-04%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%2210.2165%5C%2F00002018-199410040-00005%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%220114-5916%22%2C%22language%22%3A%22eng%22%2C%22collections%22%3A%5B%22XJF7AAVF%22%5D%2C%22dateModified%22%3A%222025-05-08T16%3A50%3A02Z%22%7D%7D%2C%7B%22key%22%3A%222H98I8KA%22%2C%22library%22%3A%7B%22id%22%3A11711645%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Mercke%20et%20al.%22%2C%22parsedDate%22%3A%222000-03%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%201.35%3B%20%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%20style%3D%26quot%3Bclear%3A%20left%3B%20%26quot%3B%26gt%3B%5Cn%20%20%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-left-margin%26quot%3B%20style%3D%26quot%3Bfloat%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%26quot%3B%26gt%3B1.%26lt%3B%5C%2Fdiv%26gt%3B%26lt%3Bdiv%20class%3D%26quot%3Bcsl-right-inline%26quot%3B%20style%3D%26quot%3Bmargin%3A%200%20.4em%200%201.5em%3B%26quot%3B%26gt%3BMercke%20Y%2C%20Sheng%20H%2C%20Khan%20T%2C%20Lippmann%20S.%20Hair%20loss%20in%20psychopharmacology.%20Ann%20Clin%20Psychiatry.%202000%20Mar%3B12%281%29%3A35%26%23x2013%3B42.%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%20%20%20%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Hair%20loss%20in%20psychopharmacology%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Y.%22%2C%22lastName%22%3A%22Mercke%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22H.%22%2C%22lastName%22%3A%22Sheng%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22T.%22%2C%22lastName%22%3A%22Khan%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22S.%22%2C%22lastName%22%3A%22Lippmann%22%7D%5D%2C%22abstractNote%22%3A%22Medication-induced%20alopecia%20is%20an%20occasional%20side%20effect%20of%20many%20psychopharmaceuticals.%20Most%20of%20the%20mood%20stabilizer%20and%20antidepressant%20drugs%20can%20lead%20to%20this%20condition.%20Some%20antipsychotic%20and%20antianxiety%20agents%20induce%20alopecia.%20Hair%20loss%20is%20also%20related%20to%20hypothyroidism%2C%20which%20can%20be%20induced%20by%20lithium%20and%20other%20agents.%20Alopecia%20might%20not%20be%20reported%20by%20some%20people%2C%20but%20physicians%20should%20be%20aware%20of%20this%20potential%20problem%20which%20may%20contribute%20to%20noncompliance.%20Lithium%20causes%20hair%20loss%20in%2012-19%25%20of%20long-term%20users.%20Valproic%20acid%20and%5C%2F%20or%20divalproex%20precipitates%20alopecia%20in%20up%20to%2012%25%20of%20patients%20in%20a%20dose-dependent%20relationship.%20Incidences%20up%20to%2028%25%20are%20observed%20with%20high%20valproate%20concentration%20exposures.%20These%20pharmaceuticals%20also%20can%20change%20hair%20color%20and%20structure.%20The%20occurrence%20of%20carbamazepine-induced%20alopecia%20is%20at%20or%20below%206%25.%20Hair%20loss%20is%20less%20common%20with%20other%20mood%20stabilizers.%20Tricyclic%20antidepressants%2C%20maprotilene%2C%20trazodone%2C%20and%20virtually%20all%20the%20new%20generation%20of%20antidepressants%20may%20on%20rare%20occasions%20lead%20to%20alopecia.%20The%20same%20applies%20to%20haloperidol%2C%20olanzepine%2C%20risperidone%2C%20clonazepam%2C%20and%20buspirone%2C%20but%20not%20to%20other%20neuroleptics%2C%20benzodiazepines%2C%20or%20barbiturates%2C%20selected%20antihistamines%2C%20and%20antiparkinsonians.%20Discontinuation%20of%20the%20medication%20or%20dose%20reduction%20almost%20always%20leads%20to%20complete%20hair%20regrowth.%20The%20therapeutic%20value%20of%20mineral%20supplements%20remains%20unclear.%22%2C%22date%22%3A%222000-03%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%2210.1023%5C%2Fa%3A1009074926921%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%221040-1237%22%2C%22language%22%3A%22eng%22%2C%22collections%22%3A%5B%22XJF7AAVF%22%5D%2C%22dateModified%22%3A%222025-05-08T16%3A48%3A26Z%22%7D%7D%2C%7B%22key%22%3A%22E3X7DJWT%22%2C%22library%22%3A%7B%22id%22%3A11711645%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Bleiker%20et%20al.%22%2C%22parsedDate%22%3A%222005-07%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%201.35%3B%20%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%20style%3D%26quot%3Bclear%3A%20left%3B%20%26quot%3B%26gt%3B%5Cn%20%20%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-left-margin%26quot%3B%20style%3D%26quot%3Bfloat%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%26quot%3B%26gt%3B1.%26lt%3B%5C%2Fdiv%26gt%3B%26lt%3Bdiv%20class%3D%26quot%3Bcsl-right-inline%26quot%3B%20style%3D%26quot%3Bmargin%3A%200%20.4em%200%201.5em%3B%26quot%3B%26gt%3BBleiker%20TO%2C%20Nicolaou%20N%2C%20Traulsen%20J%2C%20Hutchinson%20PE.%20%26%23x201C%3BAtrophic%20telogen%20effluvium%26%23x201D%3B%20from%20cytotoxic%20drugs%20and%20a%20randomized%20controlled%20trial%20to%20investigate%20the%20possible%20protective%20effect%20of%20pretreatment%20with%20a%20topical%20vitamin%20D%20analogue%20in%20humans.%20Br%20J%20Dermatol.%202005%20July%3B153%281%29%3A103%26%23x2013%3B12.%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%20%20%20%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22%27Atrophic%20telogen%20effluvium%27%20from%20cytotoxic%20drugs%20and%20a%20randomized%20controlled%20trial%20to%20investigate%20the%20possible%20protective%20effect%20of%20pretreatment%20with%20a%20topical%20vitamin%20D%20analogue%20in%20humans%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22T.%20O.%22%2C%22lastName%22%3A%22Bleiker%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22N.%22%2C%22lastName%22%3A%22Nicolaou%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22J.%22%2C%22lastName%22%3A%22Traulsen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22P.%20E.%22%2C%22lastName%22%3A%22Hutchinson%22%7D%5D%2C%22abstractNote%22%3A%22BACKGROUND%3A%20Hair%20loss%20from%20cytotoxic%20drugs%20is%20classically%20ascribed%20to%20the%20loss%20of%20fractured%20hairs%20%28anagen%20effluvium%29.%20Telogen%20hair%20loss%20has%20also%20been%20described%20but%20some%20authors%20have%20denied%20any%20effect%20on%20the%20hair%20cycle.%20There%20are%20conflicting%20reports%20on%20a%20protective%20effect%20of%20pretreatment%20with%20a%20vitamin%20D%20analogue%20on%20cytotoxic%20drug-induced%20hair%20loss%20in%20rodents.%5CnOBJECTIVES%3A%20To%20investigate%20the%20process%20of%20cytotoxic%20hair%20loss%20and%20any%20protective%20effect%20on%20the%20hair%20of%20pretreatment%20with%20topical%20calcipotriol.%5CnMETHODS%3A%20Breast%20cancer%20patients%20who%20were%20about%20to%20receive%20cycles%20of%20chemotherapy%20with%20cyclophosphamide%20600%20mg%20m%28-2%29%2C%20methotrexate%2040%20mg%20m%28-2%29%20and%205-fluorouracil%20600%20mg%20m%28-2%29%20were%20recruited%20and%20randomized%20to%20receive%20calcipotriol%20scalp%20solution%2050%20microg%20mL%28-1%29%20or%20vehicle.%20The%20solution%20was%20applied%20twice%20daily%20from%204%20days%20prior%20to%20chemotherapy%20and%20continued%20for%2014%20days%20in%20each%20treatment%20cycle.%20Shed%2C%20plucked%20and%20cut%20hairs%20were%20sampled.%20Absolute%20shed%20rates%2C%20the%20proportion%20of%20major%20hair%20types%2C%20the%20presence%20of%20proximal%20hair%20shaft%20changes%2C%20regrowth%20%28using%20the%20new%20anagen%20hair%20count%29%20and%20hair%20density%20were%20assessed.%5CnRESULTS%3A%20Ten%20patients%20receiving%20calcipotriol%20and%2014%20receiving%20vehicle%20completed%20three%20treatment%20cycles%20and%20nine%20from%20both%20groups%20completed%20six%20cycles.%20There%20was%20no%20detectable%20effect%20of%20calcipotriol%20on%20the%20proportion%20of%20patients%20experiencing%20minimal%20hair%20loss%20from%20chemotherapy%2C%20shed%20rates%2C%20plucked%20telogen%20and%20fractured%20hair%20counts%2C%20the%20morphology%20of%20shed%20and%20plucked%20hair%2C%20hair%20regrowth%20or%20hair%20density.%20Combining%20results%20of%20the%20treatment%20groups%2C%20there%20was%20a%20large%20variation%20in%20the%20impact%20of%20chemotherapy%20on%20hair%20loss%2C%20from%20total%20loss%20in%20five%20patients%20to%20no%20obvious%20loss%20in%20five.%20Excluding%20the%20latter%2C%20during%20chemotherapy%20shed%20telogen%20hairs%20%28mean%2081%25%20of%20shed%20hairs%29%20predominated%20over%20fractured%20%2812%25%29%20and%20anagen%20hairs%20%286%25%29%20%28P%20%3D%200.0002%29.%20The%20major%20pathological%20change%20was%20proximal%20hair%20shaft%20tapering%2C%20baseline%20mean%203%25%20of%20shed%20hairs%20rising%20to%2048%25%20%28P%20%3D%200.0005%29%20during%20treatment%2C%20and%20there%20was%20a%20consequent%20decrease%20in%20normal%20telogen%20hairs%2C%20baseline%20mean%2098%25%20of%20all%20telogen%20hairs%20falling%20to%2055%25%20%28P%20%3D%200.0005%29%20during%20treatment.%20The%20pathological%20tapered%20telogen%20hairs%20had%20normal%20or%20small%2C%20sometimes%20diminutive%2C%20bulbs.%20Fracturing%20of%20hairs%20with%20diminutive%20bulbs%20produced%20typical%20%26%23039%3Bexclamation%20mark%26%23039%3B%20hairs.%5CnCONCLUSIONS%3A%20The%20cardinal%20effects%20of%20cytotoxic%20drugs%20found%20in%20this%20study%20were%20tapering%20of%20the%20proximal%20hair%20shaft%20and%20premature%20entry%20of%20the%20follicle%20into%20telogen%2C%20conflicting%20with%20the%20conventional%20view%20that%20affected%20hair%20follicles%20continue%20in%20anagen.%20There%20was%20a%20resulting%20effluvium%20of%20a%20mixture%20of%20tapering%20telogen%20hairs%20and%20fractured%20hairs.%20As%20entry%20into%20telogen%20is%20an%20integral%20part%20of%20the%20process%2C%20cytotoxic%20hair%20loss%20may%20be%20regarded%20as%20a%20variant%20of%20the%20conventional%20%26%23039%3Btelogen%20effluvium%26%23039%3B%20and%20we%20propose%20the%20term%20%26%23039%3Batrophic%20telogen%20effluvium%26%23039%3B.%20There%20was%20no%20obvious%20protective%20effect%20on%20the%20hair%20loss%20of%20prior%20treatment%20with%20topical%20calcipotriol.%22%2C%22date%22%3A%222005-07%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%2210.1111%5C%2Fj.1365-2133.2005.06608.x%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%220007-0963%22%2C%22language%22%3A%22eng%22%2C%22collections%22%3A%5B%22XJF7AAVF%22%5D%2C%22dateModified%22%3A%222025-05-08T16%3A46%3A41Z%22%7D%7D%2C%7B%22key%22%3A%228U6EXSJE%22%2C%22library%22%3A%7B%22id%22%3A11711645%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Tosti%20and%20Pazzaglia%22%2C%22parsedDate%22%3A%222007-04%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%201.35%3B%20%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%20style%3D%26quot%3Bclear%3A%20left%3B%20%26quot%3B%26gt%3B%5Cn%20%20%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-left-margin%26quot%3B%20style%3D%26quot%3Bfloat%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%26quot%3B%26gt%3B1.%26lt%3B%5C%2Fdiv%26gt%3B%26lt%3Bdiv%20class%3D%26quot%3Bcsl-right-inline%26quot%3B%20style%3D%26quot%3Bmargin%3A%200%20.4em%200%201.5em%3B%26quot%3B%26gt%3BTosti%20A%2C%20Pazzaglia%20M.%20Drug%20reactions%20affecting%20hair%3A%20diagnosis.%20Dermatol%20Clin.%202007%20Apr%3B25%282%29%3A223%26%23x2013%3B31%2C%20vii.%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%20%20%20%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Drug%20reactions%20affecting%20hair%3A%20diagnosis%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antonella%22%2C%22lastName%22%3A%22Tosti%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Massimiliano%22%2C%22lastName%22%3A%22Pazzaglia%22%7D%5D%2C%22abstractNote%22%3A%22Drugs%20may%20cause%20hair%20loss%2C%20stimulate%20hair%20growth%2C%20or%20induce%20changes%20in%20the%20hair%20shape%20and%20color.%20Drug-induced%20hair%20loss%20is%2C%20in%20most%20cases%2C%20a%20consequence%20of%20a%20toxic%20effect%20of%20the%20drug%20on%20the%20hair%20matrix.%20Although%20a%20large%20number%20of%20drugs%20have%20been%20occasionally%20reported%20to%20produce%20hair%20loss%2C%20the%20relationship%20between%20drug%20intake%20and%20hair%20loss%20has%20been%20proven%20only%20for%20a%20few%20agents.%20Type%20of%20hair%20loss%20%28telogen%20effluvium%2C%20anagen%20effluvium%2C%20or%20both%29%20depends%20on%20the%20drug%2C%20its%20dosage%2C%20and%20patient%26%23039%3Bs%20susceptibility.%20Drug-induced%20hair%20loss%20is%20usually%20reversible.%22%2C%22date%22%3A%222007-04%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.det.2007.01.005%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%220733-8635%22%2C%22language%22%3A%22eng%22%2C%22collections%22%3A%5B%22XJF7AAVF%22%5D%2C%22dateModified%22%3A%222025-05-08T16%3A58%3A32Z%22%7D%7D%2C%7B%22key%22%3A%228QV6WHNT%22%2C%22library%22%3A%7B%22id%22%3A11711645%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Patel%20et%20al.%22%2C%22parsedDate%22%3A%222013-01%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%201.35%3B%20%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%20style%3D%26quot%3Bclear%3A%20left%3B%20%26quot%3B%26gt%3B%5Cn%20%20%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-left-margin%26quot%3B%20style%3D%26quot%3Bfloat%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%26quot%3B%26gt%3B1.%26lt%3B%5C%2Fdiv%26gt%3B%26lt%3Bdiv%20class%3D%26quot%3Bcsl-right-inline%26quot%3B%20style%3D%26quot%3Bmargin%3A%200%20.4em%200%201.5em%3B%26quot%3B%26gt%3BPatel%20M%2C%20Harrison%20S%2C%20Sinclair%20R.%20Drugs%20and%20hair%20loss.%20Dermatol%20Clin.%202013%20Jan%3B31%281%29%3A67%26%23x2013%3B73.%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%20%20%20%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Drugs%20and%20hair%20loss%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Mansi%22%2C%22lastName%22%3A%22Patel%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Shannon%22%2C%22lastName%22%3A%22Harrison%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Rodney%22%2C%22lastName%22%3A%22Sinclair%22%7D%5D%2C%22abstractNote%22%3A%22Hair%20loss%20is%20a%20common%20complaint%2C%20both%20in%20men%20and%20women%2C%20and%20use%20of%20prescription%20medications%20is%20widespread.%20When%20there%20is%20a%20temporal%20association%20between%20the%20onset%20of%20hair%20loss%20and%20commencement%20of%20a%20medication%2C%20the%20medication%20is%20commonly%20thought%20to%20have%20caused%20the%20hair%20loss.%20However%2C%20hair%20loss%20and%20in%20particular%20telogen%20effluvium%20may%20occur%20in%20response%20to%20a%20number%20of%20triggers%20including%20fever%2C%20hemorrhage%2C%20severe%20illness%2C%20stress%2C%20and%20childbirth%2C%20and%20a%20thorough%20exclusion%20of%20these%20potential%20confounders%20is%20necessary%20before%20the%20hair%20loss%20can%20be%20blamed%20on%20the%20medication.%20Certain%20medications%20are%20known%20to%20cause%20hair%20loss%20by%20a%20variety%20of%20mechanisms%20including%20anagen%20arrest%2C%20telogen%20effluvium%2C%20or%20accentuation%20of%20androgenetic%20alopecia%20by%20androgens.%22%2C%22date%22%3A%222013-01%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.det.2012.08.002%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%221558-0520%22%2C%22language%22%3A%22eng%22%2C%22collections%22%3A%5B%22XJF7AAVF%22%5D%2C%22dateModified%22%3A%222025-05-08T16%3A50%3A36Z%22%7D%7D%2C%7B%22key%22%3A%229SEFZ9ED%22%2C%22library%22%3A%7B%22id%22%3A11711645%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Wang%20et%20al.%22%2C%22parsedDate%22%3A%222019-07%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%201.35%3B%20%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%20style%3D%26quot%3Bclear%3A%20left%3B%20%26quot%3B%26gt%3B%5Cn%20%20%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-left-margin%26quot%3B%20style%3D%26quot%3Bfloat%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%26quot%3B%26gt%3B1.%26lt%3B%5C%2Fdiv%26gt%3B%26lt%3Bdiv%20class%3D%26quot%3Bcsl-right-inline%26quot%3B%20style%3D%26quot%3Bmargin%3A%200%20.4em%200%201.5em%3B%26quot%3B%26gt%3BWang%20X%2C%20Wang%20H%2C%20Xu%20D%2C%20Zhu%20L%2C%20Liu%20L.%20Risk%20of%20valproic%20acid-related%20alopecia%3A%20A%20systematic%20review%20and%20meta-analysis.%20Seizure.%202019%20July%3B69%3A61%26%23x2013%3B9.%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%20%20%20%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Risk%20of%20valproic%20acid-related%20alopecia%3A%20A%20systematic%20review%20and%20meta-analysis%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Xueping%22%2C%22lastName%22%3A%22Wang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Haijiao%22%2C%22lastName%22%3A%22Wang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Da%22%2C%22lastName%22%3A%22Xu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Lina%22%2C%22lastName%22%3A%22Zhu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ling%22%2C%22lastName%22%3A%22Liu%22%7D%5D%2C%22abstractNote%22%3A%22PURPOSE%3A%20We%20systematically%20reviewed%20studies%20to%20provide%20current%20evidence%20about%20the%20incidence%20and%20risk%20of%20alopecia%20in%20patients%20undergoing%20valproic%20acid%20%28VPA%29%20therapy.%5CnMETHODS%3A%20We%20retrieved%20relevant%20publications%20and%20gathered%20data%20on%20alopecia%20in%20patients%20taking%20VPA%20and%20other%20drugs%20from%20prospective%20studies.%5CnRESULTS%3A%20Twenty-five%20articles%20met%20the%20inclusion%20criteria%2C%20and%20the%20overall%20incidence%20of%20alopecia%20in%20patients%20receiving%20VPA%20therapy%20was%2011%25%20%2895%25%20confidence%20interval%20%28CI%29%3A%200.08-0.13%29.%20The%20pooled%20risk%20of%20alopecia%20showed%20a%20significant%20difference%20between%20patients%20treated%20with%20VPA%20and%20all%20other%20drugs%20%28odds%20ratio%20%28OR%29%205.02%2C%2095%25%20CI%3A%203.58-7.03%29%2C%20other%20epileptic%20drugs%20%28AEDs%29%20%28OR%204.82%2C%2095%25%20CI%3A%203.32-7.00%29%20and%20other%20non-AEDs%20%28OR%205.84%2C%2095%25%20CI%3A%202.67-12.81%29.%20Compared%20to%20other%20drugs%2C%20VPA%20increased%20the%20risk%20of%20alopecia%20both%20in%20patients%20with%20migraine%20headaches%20%28OR%206.05%2C%2095%25%20CI%3A%202.89-12.63%29%20and%20patients%20with%20epilepsy%20%28OR%205.29%2C%2095%25%20CI%3A%203.53-7.92%29%2C%20and%20the%20increase%20risk%20was%20reported%20more%20frequently%20in%20patients%20with%20migraine.%20Both%20lower%20doses%20%28OR%204.38%2C%2095%25%20CI%3A%202.32-8.25%29%20and%20shorter%20treatments%20%28OR%204.98%2C%2095%25%20CI%3A%202.41-10.25%29%20with%20VPA%20posed%20a%20high%20risk%20of%20alopecia%20compared%20to%20other%20drugs%2C%20as%20did%20higher%20doses%20and%20longer%20treatment%20times.%5CnCONCLUSIONS%3A%20Based%20on%20our%20findings%2C%20VPA%20was%20significantly%20associated%20with%20a%20risk%20of%20alopecia%20compared%20to%20other%20drugs%2C%20and%20the%20risk%20did%20not%20depend%20on%20the%20dose%20and%20treatment%20time.%22%2C%22date%22%3A%222019-07%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.seizure.2019.04.003%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%221532-2688%22%2C%22language%22%3A%22eng%22%2C%22collections%22%3A%5B%22XJF7AAVF%22%5D%2C%22dateModified%22%3A%222025-05-08T16%3A57%3A33Z%22%7D%7D%2C%7B%22key%22%3A%22EF2DDBYK%22%2C%22library%22%3A%7B%22id%22%3A11711645%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Pejcic%20and%20Paudel%22%2C%22parsedDate%22%3A%222022-07%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%201.35%3B%20%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%20style%3D%26quot%3Bclear%3A%20left%3B%20%26quot%3B%26gt%3B%5Cn%20%20%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-left-margin%26quot%3B%20style%3D%26quot%3Bfloat%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%26quot%3B%26gt%3B1.%26lt%3B%5C%2Fdiv%26gt%3B%26lt%3Bdiv%20class%3D%26quot%3Bcsl-right-inline%26quot%3B%20style%3D%26quot%3Bmargin%3A%200%20.4em%200%201.5em%3B%26quot%3B%26gt%3BPejcic%20AV%2C%20Paudel%20V.%20Alopecia%20associated%20with%20the%20use%20of%20selective%20serotonin%20reuptake%20inhibitors%3A%20Systematic%20review.%20Psychiatry%20Res.%202022%20July%3B313%3A114620.%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%20%20%20%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Alopecia%20associated%20with%20the%20use%20of%20selective%20serotonin%20reuptake%20inhibitors%3A%20Systematic%20review%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ana%20V.%22%2C%22lastName%22%3A%22Pejcic%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Vikash%22%2C%22lastName%22%3A%22Paudel%22%7D%5D%2C%22abstractNote%22%3A%22We%20aimed%20to%20systematically%20review%20published%20cases%20of%20alopecia%20%28hair%20loss%29%20associated%20with%20selective%20serotonin%20reuptake%20inhibitors%20%28SSRIs%29.%20Four%20electronic%20databases%20were%20searched%20up%20to%20November%2016%2C%202021.%20Thirty-eight%20publications%20describing%2071%20patients%20with%20a%20total%20of%2081%20episodes%20of%20alopecia%20met%20inclusion%20criteria.%20Patients%26%23039%3B%20age%20ranged%20from%207%20to%2085%20years%20and%2080.3%25%20were%20female.%20Alopecia%20most%20commonly%20affected%20scalp%20%2898.6%25%29.%20Reported%20time%20to%20onset%20ranged%20from%203%20days%20to%205%20years%20%28median%3A%208.6%20weeks%29.%20Discontinuation%20of%20the%20suspected%20SSRI%20led%20to%20recovery%20in%2063.0%25%20of%20episodes.%20Clinicians%20should%20be%20aware%20of%20this%20possible%20adverse%20effect%20of%20SSRIs.%22%2C%22date%22%3A%222022-07%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.psychres.2022.114620%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%221872-7123%22%2C%22language%22%3A%22eng%22%2C%22collections%22%3A%5B%22XJF7AAVF%22%5D%2C%22dateModified%22%3A%222025-05-08T16%3A56%3A11Z%22%7D%7D%2C%7B%22key%22%3A%22VIHUHB8H%22%2C%22library%22%3A%7B%22id%22%3A11711645%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Alhanshali%20et%20al.%22%2C%22parsedDate%22%3A%222023-08%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%201.35%3B%20%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%20style%3D%26quot%3Bclear%3A%20left%3B%20%26quot%3B%26gt%3B%5Cn%20%20%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-left-margin%26quot%3B%20style%3D%26quot%3Bfloat%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%26quot%3B%26gt%3B1.%26lt%3B%5C%2Fdiv%26gt%3B%26lt%3Bdiv%20class%3D%26quot%3Bcsl-right-inline%26quot%3B%20style%3D%26quot%3Bmargin%3A%200%20.4em%200%201.5em%3B%26quot%3B%26gt%3BAlhanshali%20L%2C%20Buontempo%20M%2C%20Shapiro%20J%2C%20Lo%20Sicco%20K.%20Medication-induced%20hair%20loss%3A%20An%20update.%20J%20Am%20Acad%20Dermatol.%202023%20Aug%3B89%282S%29%3AS20%26%23x2013%3B8.%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%20%20%20%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Medication-induced%20hair%20loss%3A%20An%20update%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Lina%22%2C%22lastName%22%3A%22Alhanshali%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Michael%22%2C%22lastName%22%3A%22Buontempo%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jerry%22%2C%22lastName%22%3A%22Shapiro%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kristen%22%2C%22lastName%22%3A%22Lo%20Sicco%22%7D%5D%2C%22abstractNote%22%3A%22This%20article%20discusses%20drug-induced%20hair%20loss%2C%20which%20can%20occur%20with%20many%20drugs%20including%20cytotoxic%20agents%2C%5Cu00a0biologics%2C%20and%20immunomodulating%20agents%2C%20among%20others.%20It%20outlines%20the%20diagnosis%20and%20management%5Cu00a0of%20drug-induced%20alopecia%2C%20with%20a%20focus%20on%20recently%20implicated%20drugs.%22%2C%22date%22%3A%222023-08%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.jaad.2023.04.022%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%221097-6787%22%2C%22language%22%3A%22eng%22%2C%22collections%22%3A%5B%22XJF7AAVF%22%5D%2C%22dateModified%22%3A%222025-05-08T16%3A42%3A45Z%22%7D%7D%2C%7B%22key%22%3A%226CE3TUXQ%22%2C%22library%22%3A%7B%22id%22%3A11711645%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Zhang%20et%20al.%22%2C%22parsedDate%22%3A%222023-12%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%201.35%3B%20%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%20style%3D%26quot%3Bclear%3A%20left%3B%20%26quot%3B%26gt%3B%5Cn%20%20%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-left-margin%26quot%3B%20style%3D%26quot%3Bfloat%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%26quot%3B%26gt%3B1.%26lt%3B%5C%2Fdiv%26gt%3B%26lt%3Bdiv%20class%3D%26quot%3Bcsl-right-inline%26quot%3B%20style%3D%26quot%3Bmargin%3A%200%20.4em%200%201.5em%3B%26quot%3B%26gt%3BZhang%20D%2C%20LaSenna%20C%2C%20Shields%20BE.%20Culprits%20of%20Medication-Induced%20Telogen%20Effluvium%2C%20Part%201.%20Cutis.%202023%20Dec%3B112%286%29%3A267%26%23x2013%3B71.%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%20%20%20%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Culprits%20of%20Medication-Induced%20Telogen%20Effluvium%2C%20Part%201%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Donglin%22%2C%22lastName%22%3A%22Zhang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Charlotte%22%2C%22lastName%22%3A%22LaSenna%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Bridget%20E.%22%2C%22lastName%22%3A%22Shields%22%7D%5D%2C%22abstractNote%22%3A%22Telogen%20effluvium%20%28TE%29%20is%20a%20common%20clinical%20consequence%20of%20medication-related%20alopecia.%20The%20inciting%20cause%20of%20TE%20may%20be%20difficult%20to%20identify%20due%20to%20delays%20in%20clinically%20apparent%20hair%20loss.%20Because%20medication-induced%20TE%20is%20a%20nonscarring%20alopecia%20that%20typically%20is%20reversible%2C%20appropriate%20management%20requires%20identification%20of%20the%20underlying%20triggering%20medication%20and%20cessation%20of%20it%2C%20if%20possible.%20In%20part%201%20of%20this%20series%2C%20we%20review%20the%20existing%20literature%20on%20medication-induced%20TE%20with%20a%20focus%20on%20systemic%20retinoids%2C%20antifungal%20agents%2C%20and%20psychotropic%20medications.%22%2C%22date%22%3A%222023-12%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%2210.12788%5C%2Fcutis.0910%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%222326-6929%22%2C%22language%22%3A%22eng%22%2C%22collections%22%3A%5B%22XJF7AAVF%22%5D%2C%22dateModified%22%3A%222025-05-08T16%3A47%3A35Z%22%7D%7D%2C%7B%22key%22%3A%22JLSI5HU6%22%2C%22library%22%3A%7B%22id%22%3A11711645%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Zhang%20et%20al.%22%2C%22parsedDate%22%3A%222024-01%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%201.35%3B%20%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%20style%3D%26quot%3Bclear%3A%20left%3B%20%26quot%3B%26gt%3B%5Cn%20%20%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-left-margin%26quot%3B%20style%3D%26quot%3Bfloat%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%26quot%3B%26gt%3B1.%26lt%3B%5C%2Fdiv%26gt%3B%26lt%3Bdiv%20class%3D%26quot%3Bcsl-right-inline%26quot%3B%20style%3D%26quot%3Bmargin%3A%200%20.4em%200%201.5em%3B%26quot%3B%26gt%3BZhang%20D%2C%20LaSenna%20C%2C%20Shields%20BE.%20Culprits%20of%20Medication-Induced%20Telogen%20Effluvium%2C%20Part%202.%20Cutis.%202024%20Jan%3B113%281%29%3A11%26%23x2013%3B4.%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%20%20%20%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Culprits%20of%20Medication-Induced%20Telogen%20Effluvium%2C%20Part%202%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Donglin%22%2C%22lastName%22%3A%22Zhang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Charlotte%22%2C%22lastName%22%3A%22LaSenna%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Bridget%20E.%22%2C%22lastName%22%3A%22Shields%22%7D%5D%2C%22abstractNote%22%3A%22Telogen%20effluvium%20%28TE%29%20is%20a%20common%20mechanism%20underlying%20medication-related%20alopecia.%20The%20inciting%20cause%20of%20TE%20may%20be%20difficult%20to%20identify%20due%20to%20delays%20in%20clinically%20apparent%20hair%20loss.%20Because%20medication-induced%20TE%20is%20a%20nonscarring%20alopecia%20that%20typically%20is%20reversible%2C%20appropriate%20management%20requires%20identification%20of%20the%20underlying%20trigger%20and%20cessation%20of%20potential%20culprit%20medications.%20In%20part%202%20of%20this%202-part%20series%20on%20medication-induced%20TE%2C%20we%20focus%20on%20anticoagulant%20and%20antihypertensive%20medications.%22%2C%22date%22%3A%222024-01%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%2210.12788%5C%2Fcutis.0919%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%222326-6929%22%2C%22language%22%3A%22eng%22%2C%22collections%22%3A%5B%22XJF7AAVF%22%5D%2C%22dateModified%22%3A%222025-05-08T16%3A41%3A30Z%22%7D%7D%2C%7B%22key%22%3A%22AJ59ZGVS%22%2C%22library%22%3A%7B%22id%22%3A11711645%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Hill%20et%20al.%22%2C%22parsedDate%22%3A%222025-02%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%201.35%3B%20%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%20style%3D%26quot%3Bclear%3A%20left%3B%20%26quot%3B%26gt%3B%5Cn%20%20%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-left-margin%26quot%3B%20style%3D%26quot%3Bfloat%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%26quot%3B%26gt%3B1.%26lt%3B%5C%2Fdiv%26gt%3B%26lt%3Bdiv%20class%3D%26quot%3Bcsl-right-inline%26quot%3B%20style%3D%26quot%3Bmargin%3A%200%20.4em%200%201.5em%3B%26quot%3B%26gt%3BHill%20RC%2C%20Zeldin%20SD%2C%20Lipner%20SR.%20Drug-Induced%20Hair%20Loss%3A%20Analysis%20of%20the%20Food%20and%20Drug%20Administration%26%23x2019%3Bs%20Adverse%20Events%20Reporting%20System%20Database.%20Skin%20Appendage%20Disord.%202025%20Feb%3B11%281%29%3A63%26%23x2013%3B9.%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%20%20%20%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Drug-Induced%20Hair%20Loss%3A%20Analysis%20of%20the%20Food%20and%20Drug%20Administration%27s%20Adverse%20Events%20Reporting%20System%20Database%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Rachel%20C.%22%2C%22lastName%22%3A%22Hill%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Steven%20D.%22%2C%22lastName%22%3A%22Zeldin%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Shari%20R.%22%2C%22lastName%22%3A%22Lipner%22%7D%5D%2C%22abstractNote%22%3A%22INTRODUCTION%3A%20Drug-induced%20hair%20loss%20is%20one%20of%20several%20causes%20of%20hair%20loss%20commonly%20seen%20in%20clinical%20practice%2C%20and%20it%20is%20often%20a%20daunting%20task%20to%20determine%20a%20potential%20culprit%20drug%20when%20a%20patient%20is%20taking%20numerous%20medications.%20Our%20objective%20was%20to%20identify%20drugs%20responsible%20for%20hair%20loss%2C%20using%20the%20Food%20and%20Drug%20Administration%26%23039%3Bs%20Adverse%20Events%20Reporting%20System%20%28FAERS%29%20database%2C%20a%20compilation%20of%20drug-related%20adverse%20events%20%28AEs%29.%5CnMETHODS%3A%20Using%20the%20FAERS%20database%2C%20we%20queried%20all%20domestic%20reports%20with%20the%20reaction%20term%20%26quot%3Balopecia%26quot%3B%20listed%20as%20an%20AE%20in%20patients%20%5Cu226518%20years%20old%20from%20April%201%2C%201968%2C%20to%20September%2029%2C%202023.%20Using%20descriptive%20statistics%2C%20individual%20agents%20were%20grouped%20by%20drug%20class.%5CnRESULTS%3A%20We%20analyzed%20a%20total%20of%2039%2C346%20hair%20loss%20AE%20reports%20related%20to%20a%20single%20agent.%20Immunomodulatory%20agents%20and%20monoclonal%20antibodies%20represented%20the%20highest%20proportion%20of%20AE%20reports%20for%20alopecia%2C%20followed%20by%20hair%20loss%20drugs%2C%20contraceptives%2C%20and%20antitumor%20necrosis%20factor%20%28anti-TNF%29%20biologics.%5CnCONCLUSION%3A%20In%20sum%2C%20we%20showed%20that%20immunomodulatory%20agents%20and%20monoclonal%20antibodies%2C%20hair%20loss%20drugs%2C%20including%20minoxidil%20and%20finasteride%2C%20contraceptives%2C%20kinase%20inhibitors%2C%20and%20anti-TNF%20drugs%20are%20most%20frequently%20associated%20with%20hair%20loss%20AEs%20in%20the%20FAERS%20database.%20Because%20many%20of%20these%20drugs%20are%20not%20prescribed%20primarily%20for%20dermatologic%20indications%2C%20our%20study%20provides%20guidance%20for%20dermatologists%20in%20identifying%20common%20medications%20associated%20with%20alopecia.%22%2C%22date%22%3A%222025-02%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%2210.1159%5C%2F000540104%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%222296-9195%22%2C%22language%22%3A%22eng%22%2C%22collections%22%3A%5B%22XJF7AAVF%22%5D%2C%22dateModified%22%3A%222025-05-08T16%3A44%3A08Z%22%7D%7D%5D%7D 1.
Tosi A, Misciali C, Piraccini BM, Peluso AM, Bardazzi F. Drug-induced hair loss and hair growth. Incidence, management and avoidance. Drug Saf. 1994 Apr;10(4):310–7.
1.
Mercke Y, Sheng H, Khan T, Lippmann S. Hair loss in psychopharmacology. Ann Clin Psychiatry. 2000 Mar;12(1):35–42.
1.
Bleiker TO, Nicolaou N, Traulsen J, Hutchinson PE. “Atrophic telogen effluvium” from cytotoxic drugs and a randomized controlled trial to investigate the possible protective effect of pretreatment with a topical vitamin D analogue in humans. Br J Dermatol. 2005 July;153(1):103–12.
1.
Tosti A, Pazzaglia M. Drug reactions affecting hair: diagnosis. Dermatol Clin. 2007 Apr;25(2):223–31, vii.
1.
Patel M, Harrison S, Sinclair R. Drugs and hair loss. Dermatol Clin. 2013 Jan;31(1):67–73.
1.
Wang X, Wang H, Xu D, Zhu L, Liu L. Risk of valproic acid-related alopecia: A systematic review and meta-analysis. Seizure. 2019 July;69:61–9.
1.
Pejcic AV, Paudel V. Alopecia associated with the use of selective serotonin reuptake inhibitors: Systematic review. Psychiatry Res. 2022 July;313:114620.
1.
Alhanshali L, Buontempo M, Shapiro J, Lo Sicco K. Medication-induced hair loss: An update. J Am Acad Dermatol. 2023 Aug;89(2S):S20–8.
1.
Zhang D, LaSenna C, Shields BE. Culprits of Medication-Induced Telogen Effluvium, Part 1. Cutis. 2023 Dec;112(6):267–71.
1.
Zhang D, LaSenna C, Shields BE. Culprits of Medication-Induced Telogen Effluvium, Part 2. Cutis. 2024 Jan;113(1):11–4.
1.
Hill RC, Zeldin SD, Lipner SR. Drug-Induced Hair Loss: Analysis of the Food and Drug Administration’s Adverse Events Reporting System Database. Skin Appendage Disord. 2025 Feb;11(1):63–9.